The Urinary System: Part A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Kidney, Renal Tubule – Dilation
Kidney, Renal Tubule – Dilation Figure Legend: Figure 1 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Dilated tubules are noted as tracts running through the cortex and outer medulla. Figure 2 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is present throughout the outer stripe of the outer medulla, extending into the cortex. Figure 3 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Slight tubule dilation is associated with degeneration and necrosis. Figure 4 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is associated with chronic progressive nephropathy. Comment: Renal tubule dilation may occur anywhere along the nephron or collecting duct system. It may occur in focal areas or as tracts running along the entire length of kidney sections (Figure 1). 1 Kidney, Renal Tubule – Dilation Renal tubule dilation may occur from xenobiotic administration, secondary mechanisms, or an unknown pathogenesis (see Kidney – Nephropathy, Obstructive (Figure 2). Dilation may result from direct toxic injury to the tubule epithelium interfering with absorption and secretion (Figure 3). It may also occur secondary to renal ischemia or from prolonged diuresis related to drug administration. Secondary mechanisms of tubule dilation may result from lower urinary tract obstruction, the deposition of tubule crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy (Figure 4). A few dilated tubules may be regarded as normal histologic variation. Recommendation: Renal tubule dilation should be diagnosed and given a severity grade. The location of tubule dilation should be included in the diagnosis as a site modifier. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Excretory Products and Their Elimination
290 BIOLOGY CHAPTER 19 EXCRETORY PRODUCTS AND THEIR ELIMINATION 19.1 Human Animals accumulate ammonia, urea, uric acid, carbon dioxide, water Excretory and ions like Na+, K+, Cl–, phosphate, sulphate, etc., either by metabolic System activities or by other means like excess ingestion. These substances have to be removed totally or partially. In this chapter, you will learn the 19.2 Urine Formation mechanisms of elimination of these substances with special emphasis on 19.3 Function of the common nitrogenous wastes. Ammonia, urea and uric acid are the major Tubules forms of nitrogenous wastes excreted by the animals. Ammonia is the most toxic form and requires large amount of water for its elimination, 19.4 Mechanism of whereas uric acid, being the least toxic, can be removed with a minimum Concentration of loss of water. the Filtrate The process of excreting ammonia is Ammonotelism. Many bony fishes, 19.5 Regulation of aquatic amphibians and aquatic insects are ammonotelic in nature. Kidney Function Ammonia, as it is readily soluble, is generally excreted by diffusion across 19.6 Micturition body surfaces or through gill surfaces (in fish) as ammonium ions. Kidneys do not play any significant role in its removal. Terrestrial adaptation 19.7 Role of other necessitated the production of lesser toxic nitrogenous wastes like urea Organs in and uric acid for conservation of water. Mammals, many terrestrial Excretion amphibians and marine fishes mainly excrete urea and are called ureotelic 19.8 Disorders of the animals. Ammonia produced by metabolism is converted into urea in the Excretory liver of these animals and released into the blood which is filtered and System excreted out by the kidneys. -

Nitric Oxide Synthase in Macula Densa Regulates Glomerular Capillary
Proc. Nati. Acad. Sci. USA Vol. 89, pp. 11993-11997, December 1992 Pharmacology Nitric oxide synthase in macula densa regulates glomerular capillary pressure (kidney/tubuloglomerular feedback response/glomerular ifitration rate/afferent arteriole) CHRISTOPHER S. WILCOX*t, WILLIAM J. WELCH*, FERID MURADf, STEVEN S. GROSS§, GRAHAM TAYLOR¶, ROBERTO LEVI§, AND HARALD H. H. W. SCHMIDTII** *Division of Nephrology, Hypertension and Transplantation Departments of Medicine, Pharmacology and Therapeutics, University of Florida College of Medicine and Department of Veterans Affairs Medical Center, Gainesville, FL 32608; I'Department of Pharmacology, Northwestern University School of Medicine, Chicago, IL; tAbbott Laboratories, Abbott Park, IL 60064-3500; iDepartment of Pharmacology, Cornell University Medical College, New York, NY 10021; and IDepartment of Clinical Pharmacology, The Royal Postgraduate Medical School, Hammersmith Hospital, London, England W12 OHS Communicated by Robert F. Furchgott, September 3, 1992 ABSTRACT Tubular-fluid reabsorption by specialized Previous studies have established that L-arginine-derived cells of the nephron at the junction of the ascending limb of the nitric oxide (NO) is produced by several cells within the loop of Henle and the distal convoluted tubule, termed the kidney, including isolated glomerular mesangial (6) and en- macula densa, releases compounds causing vasoconstriction of dothelial cells (7), and a renal epithelial cell line (8), but its the adjacent afferent arteriole. Activation of this tubuloglo- integrative role in the control ofrenal function is not yet clear merular feedback response reduces glomerular capillary pres- (9). In the vessel wall, the endothelium can mediate vasodi- sure of the nephron and, hence, the glomerular filtration rate. lator responses to agents such as acetylcholine (10) and can The tubuloglomerular feedback response functions in a nega- blunt the actions of certain vasoconstrictors (11). -

Basic Histology (23 Questions): Oral Histology (16 Questions
Board Question Breakdown (Anatomic Sciences section) The Anatomic Sciences portion of part I of the Dental Board exams consists of 100 test items. They are broken up into the following distribution: Gross Anatomy (50 questions): Head - 28 questions broken down in this fashion: - Oral cavity - 6 questions - Extraoral structures - 12 questions - Osteology - 6 questions - TMJ and muscles of mastication - 4 questions Neck - 5 questions Upper Limb - 3 questions Thoracic cavity - 5 questions Abdominopelvic cavity - 2 questions Neuroanatomy (CNS, ANS +) - 7 questions Basic Histology (23 questions): Ultrastructure (cell organelles) - 4 questions Basic tissues - 4 questions Bone, cartilage & joints - 3 questions Lymphatic & circulatory systems - 3 questions Endocrine system - 2 questions Respiratory system - 1 question Gastrointestinal system - 3 questions Genitouirinary systems - (reproductive & urinary) 2 questions Integument - 1 question Oral Histology (16 questions): Tooth & supporting structures - 9 questions Soft oral tissues (including dentin) - 5 questions Temporomandibular joint - 2 questions Developmental Biology (11 questions): Osteogenesis (bone formation) - 2 questions Tooth development, eruption & movement - 4 questions General embryology - 2 questions 2 National Board Part 1: Review questions for histology/oral histology (Answers follow at the end) 1. Normally most of the circulating white blood cells are a. basophilic leukocytes b. monocytes c. lymphocytes d. eosinophilic leukocytes e. neutrophilic leukocytes 2. Blood platelets are products of a. osteoclasts b. basophils c. red blood cells d. plasma cells e. megakaryocytes 3. Bacteria are frequently ingested by a. neutrophilic leukocytes b. basophilic leukocytes c. mast cells d. small lymphocytes e. fibrocytes 4. It is believed that worn out red cells are normally destroyed in the spleen by a. neutrophils b. -
Urinary System
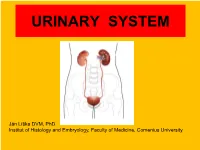
URINARY SYSTEM Ján Líška DVM, PhD Institut of Histology and Embryology, Faculty of Medicine, Comenius University Urinary system • The kidneys are the organ with multiple functions: • filtration of the blood • excretion of metabolic waste products and related removal of toxins • maintenance blood volume • regulation of acid-base balance • regulation of fluid and electrolyte balance • production of the hormones The other components of urinary system are accessory. Their function is essentially in order to eliminate urine. Urinary system - anatomy • Kidney are located in the retroperitoneal space • The surface of the kidney is covered by a fibrous capsule of dense connective tissue. • This capsule is coated with adipose capsule. • Each kidney is attached to a ureter, which carries urine to the bladder and urine is discharged out through the urethra. ANATOMIC STRUCTURE OF THE KIDNEY RENAL LOBES CORTEX outer shell columns Excretory portion medullary rays MEDULLA medullary pyramids HILUM Collecting system blood vessels lymph vessels major calyces nerves RENAL PELVIS minor calyces ureter Cortex is the outer layer surrounding the internal medulla. The cortex contains renal corpuscles, convoluted parts of prox. and dist. tubules. Renal column: the renal tissue projection between two medullary pyramids which supports the cortex. Renal pyramids: the conical segments within the medulla. They contain the ductal apparatus and stright parts of the tubules. They posses papilla - having openings through which urine passes into the calyces. Each pyramid together with the associated overlying cortex forms a renal lobe. renal pyramid papilla minor calix minor calyx Medullary rays: are in the middle of cortical part of the renal lobe, consisting of a group of the straight portiones of nephrons and the collec- medullary rays ting tubules (only straight tubules). -
The Urinary Tract and How It Works
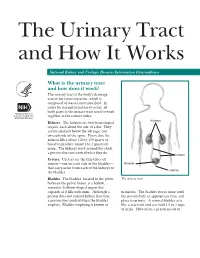
The Urinary Tract and How It Works National Kidney and Urologic Diseases Information Clearinghouse What is the urinary tract and how does it work? The urinary tract is the body’s drainage system for removing urine, which is composed of wastes and extra fluid. In order for normal urination to occur, all body parts in the urinary tract need to work together in the correct order. Kidneys Kidneys. The kidneys are two bean-shaped organs, each about the size of a fist. They are located just below the rib cage, one on each side of the spine. Every day, the kidneys filter about 120 to 150 quarts of blood to produce about 1 to 2 quarts of urine. The kidneys work around the clock; a person does not control what they do. Ureters Ureters. Ureters are the thin tubes of muscle—one on each side of the bladder— Bladder that carry urine from each of the kidneys to Urethra the bladder. Bladder. The bladder, located in the pelvis The urinary tract between the pelvic bones, is a hollow, muscular, balloon-shaped organ that expands as it fills with urine. Although a urination. The bladder stores urine until person does not control kidney function, the person finds an appropriate time and a person does control when the bladder place to urinate. A normal bladder acts empties. Bladder emptying is known as like a reservoir and can hold 1.5 to 2 cups of urine. How often a person needs to urinate depends on how quickly the kidneys Why is the urinary tract produce the urine that fills the bladder. -

Claudins in the Renal Collecting Duct
International Journal of Molecular Sciences Review Claudins in the Renal Collecting Duct Janna Leiz 1,2 and Kai M. Schmidt-Ott 1,2,3,* 1 Department of Nephrology and Intensive Care Medicine, Charité-Universitätsmedizin Berlin, 12203 Berlin, Germany; [email protected] 2 Molecular and Translational Kidney Research, Max-Delbrück-Center for Molecular Medicine in the Helmholtz Association (MDC), 13125 Berlin, Germany 3 Berlin Institute of Health (BIH), 10178 Berlin, Germany * Correspondence: [email protected]; Tel.: +49-(0)30-450614671 Received: 22 October 2019; Accepted: 20 December 2019; Published: 28 December 2019 Abstract: The renal collecting duct fine-tunes urinary composition, and thereby, coordinates key physiological processes, such as volume/blood pressure regulation, electrolyte-free water reabsorption, and acid-base homeostasis. The collecting duct epithelium is comprised of a tight epithelial barrier resulting in a strict separation of intraluminal urine and the interstitium. Tight junctions are key players in enforcing this barrier and in regulating paracellular transport of solutes across the epithelium. The features of tight junctions across different epithelia are strongly determined by their molecular composition. Claudins are particularly important structural components of tight junctions because they confer barrier and transport properties. In the collecting duct, a specific set of claudins (Cldn-3, Cldn-4, Cldn-7, Cldn-8) is expressed, and each of these claudins has been implicated in mediating aspects of the specific properties of its tight junction. The functional disruption of individual claudins or of the overall barrier function results in defects of blood pressure and water homeostasis. In this concise review, we provide an overview of the current knowledge on the role of the collecting duct epithelial barrier and of claudins in collecting duct function and pathophysiology. -

Renal Corpuscle Renal System > Histology > Histology
Renal Corpuscle Renal System > Histology > Histology Key Points: • The renal corpuscles lie within the renal cortex; • They comprise the glomerular, aka, Bowman's capsule and capillaries The capsule is a double-layer sac of epithelium: — The outer parietal layer folds upon itself to form the visceral layer. — The inner visceral layer envelops the glomerular capillaries. • As blood passes through the glomerular capillaries, aka, glomerulus, specific components, including water and wastes, are filtered to create ultrafiltrate. • The filtration barrier, which determines ultrafiltrate composition, comprises glomerular capillary endothelia, a basement membrane, and the visceral layer of the glomerular capsule. • Nephron tubules modify the ultrafiltrate to form urine. Overview Diagram: • Tuft of glomerular capillaries; blood enters the capillaries via the afferent arteriole, and exits via efferent arteriole. • The visceral layer of the glomerular capsule envelops the capillaries, then folds outwards to become the parietal layer. • The capsular space lies between the parietal and visceral layers; this space fills with ultrafiltrate. • Vascular pole = where the arterioles pass through the capsule • Urinary pole = where the nephron tubule begins • Distal tubule passes by the afferent arteriole. Details of Capillary and Visceral Layer: • Fenestrated glomerular capillary; fenestrations are small openings, aka, pores, in the endothelium that confer permeability. • Thick basement membrane overlies capillaries • Visceral layer comprises podocytes: — Cell bodies — Cytoplasmic extensions, called primary processes, give rise to secondary foot processes, aka, pedicles. • The pedicles interdigitate to form filtration slits; molecules pass through these slits to form the ultrafiltrate in the 1 / 3 capsular space. • Subpodocyte space; healthy podocytes do not adhere to the basement membrane. Clinical Correlation: • Podocyte injury causes dramatic changes in shape, and, therefore, their ability to filter substances from the blood. -

Kidney Function • Filtration • Reabsorption • Secretion • Excretion • Micturition
About This Chapter • Functions of the kidneys • Anatomy of the urinary system • Overview of kidney function • Filtration • Reabsorption • Secretion • Excretion • Micturition © 2016 Pearson Education, Inc. Functions of the Kidneys • Regulation of extracellular fluid volume and blood pressure • Regulation of osmolarity • Maintenance of ion balance • Homeostatic regulation of pH • Excretion of wastes • Production of hormones © 2016 Pearson Education, Inc. Anatomy of the Urinary System • Kidneys, ureters, bladder, and urethra • Kidneys – Bean-shaped organ – Cortex and medulla © 2016 Pearson Education, Inc. Anatomy of the Urinary System • Functional unit is the nephron – Glomerulus in the Bowman’s capsule – Proximal tubule – The loop of Henle • Descending limb and ascending limb twisted between arterioles forming the juxtaglomerular apparatus – Distal tubule – Collecting ducts © 2016 Pearson Education, Inc. Figure 19.1b Anatomy summary The kidneys are located retroperitoneally at the level of the lower ribs. Inferior Diaphragm vena cava Aorta Left adrenal gland Left kidney Right kidney Renal artery Renal vein Ureter Peritoneum Urinary Rectum (cut) bladder (cut) © 2016 Pearson Education, Inc. Figure 19.1c Anatomy summary © 2016 Pearson Education, Inc. Figure 19.1d Anatomy summary © 2016 Pearson Education, Inc. Figure 19.1f-h Anatomy summary Some nephrons dip deep into the medulla. One nephron has two arterioles and two sets of capillaries that form a portal system. Efferent arteriole Arterioles Peritubular Juxtaglomerular capillaries The cortex apparatus contains all Bowman’s Nephrons Afferent capsules, arteriole Glomerulus proximal Juxtamedullary nephron and distal (capillaries) with vasa recta tubules. Peritubular capillaries Glomerulus The medulla contains loops of Henle and Vasa recta collecting ducts. Collecting duct Loop of Henle © 2016 Pearson Education, Inc. -

Urinary System
OUTLINE 27.1 General Structure and Functions of the Urinary System 818 27.2 Kidneys 820 27 27.2a Gross and Sectional Anatomy of the Kidney 820 27.2b Blood Supply to the Kidney 821 27.2c Nephrons 824 27.2d How Tubular Fluid Becomes Urine 828 27.2e Juxtaglomerular Apparatus 828 Urinary 27.2f Innervation of the Kidney 828 27.3 Urinary Tract 829 27.3a Ureters 829 27.3b Urinary Bladder 830 System 27.3c Urethra 833 27.4 Aging and the Urinary System 834 27.5 Development of the Urinary System 835 27.5a Kidney and Ureter Development 835 27.5b Urinary Bladder and Urethra Development 835 MODULE 13: URINARY SYSTEM mck78097_ch27_817-841.indd 817 2/25/11 2:24 PM 818 Chapter Twenty-Seven Urinary System n the course of carrying out their specific functions, the cells Besides removing waste products from the bloodstream, the uri- I of all body systems produce waste products, and these waste nary system performs many other functions, including the following: products end up in the bloodstream. In this case, the bloodstream is ■ Storage of urine. Urine is produced continuously, but analogous to a river that supplies drinking water to a nearby town. it would be quite inconvenient if we were constantly The river water may become polluted with sediment, animal waste, excreting urine. The urinary bladder is an expandable, and motorboat fuel—but the town has a water treatment plant that muscular sac that can store as much as 1 liter of urine. removes these waste products and makes the water safe to drink.