Circulating Testican-2 Is a Podocyte-Derived Marker of Kidney Health
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Kidney, Renal Tubule – Dilation
Kidney, Renal Tubule – Dilation Figure Legend: Figure 1 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Dilated tubules are noted as tracts running through the cortex and outer medulla. Figure 2 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is present throughout the outer stripe of the outer medulla, extending into the cortex. Figure 3 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Slight tubule dilation is associated with degeneration and necrosis. Figure 4 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is associated with chronic progressive nephropathy. Comment: Renal tubule dilation may occur anywhere along the nephron or collecting duct system. It may occur in focal areas or as tracts running along the entire length of kidney sections (Figure 1). 1 Kidney, Renal Tubule – Dilation Renal tubule dilation may occur from xenobiotic administration, secondary mechanisms, or an unknown pathogenesis (see Kidney – Nephropathy, Obstructive (Figure 2). Dilation may result from direct toxic injury to the tubule epithelium interfering with absorption and secretion (Figure 3). It may also occur secondary to renal ischemia or from prolonged diuresis related to drug administration. Secondary mechanisms of tubule dilation may result from lower urinary tract obstruction, the deposition of tubule crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy (Figure 4). A few dilated tubules may be regarded as normal histologic variation. Recommendation: Renal tubule dilation should be diagnosed and given a severity grade. The location of tubule dilation should be included in the diagnosis as a site modifier. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Instability Restricts Signaling of Multiple Fibroblast Growth Factors
Cell. Mol. Life Sci. DOI 10.1007/s00018-015-1856-8 Cellular and Molecular Life Sciences RESEARCH ARTICLE Instability restricts signaling of multiple fibroblast growth factors Marcela Buchtova • Radka Chaloupkova • Malgorzata Zakrzewska • Iva Vesela • Petra Cela • Jana Barathova • Iva Gudernova • Renata Zajickova • Lukas Trantirek • Jorge Martin • Michal Kostas • Jacek Otlewski • Jiri Damborsky • Alois Kozubik • Antoni Wiedlocha • Pavel Krejci Received: 18 June 2014 / Revised: 7 February 2015 / Accepted: 9 February 2015 Ó Springer Basel 2015 Abstract Fibroblast growth factors (FGFs) deliver ex- failure to activate FGF receptor signal transduction over tracellular signals that govern many developmental and long periods of time, and influence specific cell behavior regenerative processes, but the mechanisms regulating FGF in vitro and in vivo. Stabilization via exogenous heparin signaling remain incompletely understood. Here, we ex- binding, introduction of stabilizing mutations or lowering plored the relationship between intrinsic stability of FGF the cell cultivation temperature rescues signaling of un- proteins and their biological activity for all 18 members of stable FGFs. Thus, the intrinsic ligand instability is an the FGF family. We report that FGF1, FGF3, FGF4, FGF6, important elementary level of regulation in the FGF sig- FGF8, FGF9, FGF10, FGF16, FGF17, FGF18, FGF20, and naling system. FGF22 exist as unstable proteins, which are rapidly de- graded in cell cultivation media. Biological activity of Keywords Fibroblast growth factor Á FGF Á Unstable Á FGF1, FGF3, FGF4, FGF6, FGF8, FGF10, FGF16, FGF17, Proteoglycan Á Regulation and FGF20 is limited by their instability, manifesting as Electronic supplementary material The online version of this article (doi:10.1007/s00018-015-1856-8) contains supplementary material, which is available to authorized users. -

Expression of Fibroblast Growth Factor 9 in Normal Human Lung and Idiopathic Pulmonary Fibrosis
JHCXXX10.1369/0022155413497366Coffey et al.FGF9 in IPF 497366research-article2013 Article Journal of Histochemistry & Cytochemistry 61(9) 671 –679 © The Author(s) 2013 Reprints and permissions: sagepub.com/journalsPermissions.nav DOI: 10.1369/0022155413497366 jhc.sagepub.com Expression of Fibroblast Growth Factor 9 in Normal Human Lung and Idiopathic Pulmonary Fibrosis Emily Coffey, Donna R. Newman, and Philip L. Sannes Department of Molecular Biomedical Sciences, Center for Comparative Medicine and Translational Research, College of Veterinary Medicine, North Carolina State University, Raleigh, North Carolina Summary The fibroblast growth factor (FGF) family of signaling ligands contributes significantly to lung development and maintenance in the adult. FGF9 is involved in control of epithelial branching and mesenchymal proliferation and expansion in developing lungs. However, its activity and expression in the normal adult lung and by epithelial and interstitial cells in fibroproliferative diseases like idiopathic pulmonary fibrosis (IPF) are unknown. Tissue samples from normal organ donor human lungs and those of a cohort of patients with mild to severe IPF were sectioned and stained for the immunolocalization of FGF9. In normal lungs, FGF9 was confined to smooth muscle surrounding airways, alveolar ducts and sacs, and blood vessels. In addition to these same sites, lungs of IPF patients expressed FGF9 in a population of myofibroblasts within fibroblastic foci, hypertrophic and hyperplastic epithelium of airways and alveoli, and smooth muscle cells surrounding vessels embedded in thickened interstitium. The results demonstrate that FGF9 protein increased in regions of active cellular hyperplasia, metaplasia, and fibrotic expansion of IPF lungs, and in isolated human lung fibroblasts treated with TGF- β1 and/or overexpressing Wnt7B. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Plakophilin-2 Haploinsufficiency Causes Calcium Handling
International Journal of Molecular Sciences Article Plakophilin-2 Haploinsufficiency Causes Calcium Handling Deficits and Modulates the Cardiac Response Towards Stress Chantal J.M. van Opbergen 1 , Maartje Noorman 1, Anna Pfenniger 2, Jaël S. Copier 1, Sarah H. Vermij 2,3 , Zhen Li 2, Roel van der Nagel 1, Mingliang Zhang 2, Jacques M.T. de Bakker 1,4, Aaron M. Glass 5, Peter J. Mohler 6,7, Steven M. Taffet 5, Marc A. Vos 1, Harold V.M. van Rijen 1, Mario Delmar 2 and Toon A.B. van Veen 1,* 1 Department of Medical Physiology, Division of Heart & Lungs, University Medical Center Utrecht, Yalelaan 50, 3584CM Utrecht, The Netherlands 2 Division of Cardiology, NYU School of Medicine, New York, NY 10016, USA 3 Institute of Biochemistry and Molecular Medicine, University of Bern, 3012 Bern, Switzerland 4 Department of Medical Biology, Academic Medical Center Amsterdam, 1105AZ Amsterdam, The Netherlands 5 Department of Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, NY 13210, USA 6 Dorothy M. Davis Heart and Lung Research Institute, The Ohio State University College of Medicine and Wexner Medical Center, Columbus, OH 43210, USA 7 Departments of Physiology & Cell Biology and Internal Medicine, Division of Cardiovascular Medicine, The Ohio State University College of Medicine Wexner Medical Center, Columbus, OH 43210, USA * Correspondence: [email protected] Received: 1 August 2019; Accepted: 19 August 2019; Published: 21 August 2019 Abstract: Human variants in plakophilin-2 (PKP2) associate with most cases of familial arrhythmogenic cardiomyopathy (ACM). Recent studies show that PKP2 not only maintains intercellular coupling, but also regulates transcription of genes involved in Ca2+ cycling and cardiac rhythm. -

FGF Signaling Regulates Mesenchymal Differentiation and Skeletal Patterning Along the Limb Bud Proximodistal Axis Kai Yu and David M
RESEARCH ARTICLE 483 Development 135, 483-491 (2008) doi:10.1242/dev.013268 FGF signaling regulates mesenchymal differentiation and skeletal patterning along the limb bud proximodistal axis Kai Yu and David M. Ornitz* Fibroblast growth factors (FGFs) are signals from the apical ectodermal ridge (AER) that are essential for limb pattern formation along the proximodistal (PD) axis. However, how patterning along the PD axis is regulated by AER-FGF signals remains controversial. To further explore the molecular mechanism of FGF functions during limb development, we conditionally inactivated fgf receptor 2 (Fgfr2) in the mouse AER to terminate all AER functions; for comparison, we inactivated both Fgfr1 and Fgfr2 in limb mesenchyme to block mesenchymal AER-FGF signaling. We also re-examined published data in which Fgf4 and Fgf8 were inactivated in the AER. We conclude that limb skeletal phenotypes resulting from loss of AER-FGF signals cannot simply be a consequence of excessive mesenchymal cell death, as suggested by previous studies, but also must be a consequence of reduced mesenchymal proliferation and a failure of mesenchymal differentiation, which occur following loss of both Fgf4 and Fgf8. We further conclude that chondrogenic primordia formation, marked by initial Sox9 expression in limb mesenchyme, is an essential component of the PD patterning process and that a key role for AER-FGF signaling is to facilitate SOX9 function and to ensure progressive establishment of chondrogenic primordia along the PD axis. KEY WORDS: FGF, FGF receptor, Apical ectodermal ridge (AER), Limb bud development, Chondrogenesis INTRODUCTION Previous studies indicate that AER-FGF signals are important The apical ectodermal ridge (AER) is a specialized ectodermal for maintaining mesenchymal cell survival during limb structure formed at the distal tip of the vertebrate limb bud that is development (Boulet et al., 2004; Sun et al., 2002). -

FGF Signaling Network in the Gastrointestinal Tract (Review)
163-168 1/6/06 16:12 Page 163 INTERNATIONAL JOURNAL OF ONCOLOGY 29: 163-168, 2006 163 FGF signaling network in the gastrointestinal tract (Review) MASUKO KATOH1 and MASARU KATOH2 1M&M Medical BioInformatics, Hongo 113-0033; 2Genetics and Cell Biology Section, National Cancer Center Research Institute, Tokyo 104-0045, Japan Received March 29, 2006; Accepted May 2, 2006 Abstract. Fibroblast growth factor (FGF) signals are trans- Contents duced through FGF receptors (FGFRs) and FRS2/FRS3- SHP2 (PTPN11)-GRB2 docking protein complex to SOS- 1. Introduction RAS-RAF-MAPKK-MAPK signaling cascade and GAB1/ 2. FGF family GAB2-PI3K-PDK-AKT/aPKC signaling cascade. The RAS~ 3. Regulation of FGF signaling by WNT MAPK signaling cascade is implicated in cell growth and 4. FGF signaling network in the stomach differentiation, the PI3K~AKT signaling cascade in cell 5. FGF signaling network in the colon survival and cell fate determination, and the PI3K~aPKC 6. Clinical application of FGF signaling cascade in cell polarity control. FGF18, FGF20 and 7. Clinical application of FGF signaling inhibitors SPRY4 are potent targets of the canonical WNT signaling 8. Perspectives pathway in the gastrointestinal tract. SPRY4 is the FGF signaling inhibitor functioning as negative feedback apparatus for the WNT/FGF-dependent epithelial proliferation. 1. Introduction Recombinant FGF7 and FGF20 proteins are applicable for treatment of chemotherapy/radiation-induced mucosal injury, Fibroblast growth factor (FGF) family proteins play key roles while recombinant FGF2 protein and FGF4 expression vector in growth and survival of stem cells during embryogenesis, are applicable for therapeutic angiogenesis. Helicobacter tissues regeneration, and carcinogenesis (1-4). -

Age-Driven Developmental Drift in the Pathogenesis of Idiopathic Pulmonary Fibrosis
BACK TO BASICS INTERSTITIAL LUNG DISEASES | Age-driven developmental drift in the pathogenesis of idiopathic pulmonary fibrosis Moisés Selman1, Carlos López-Otín2 and Annie Pardo3 Affiliations: 1Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico city, Mexico. 2Departamento de Bioquímica y Biología Molecular, Facultad de Medicina, Instituto Universitario de Oncología, Universidad de Oviedo, Oviedo, Spain. 3Facultad de Ciencias, Universidad Nacional Autónoma de México, Mexico city, Mexico. Correspondence: Moisés Selman, Instituto Nacional de Enfermedades Respiratorias, Tlalpan 4502, CP 14080, México DF, México. E-mail: [email protected] ABSTRACT Idiopathic pulmonary fibrosis (IPF) is a progressive and usually lethal disease of unknown aetiology. A growing body of evidence supports that IPF represents an epithelial-driven process characterised by aberrant epithelial cell behaviour, fibroblast/myofibroblast activation and excessive accumulation of extracellular matrix with the subsequent destruction of the lung architecture. The mechanisms involved in the abnormal hyper-activation of the epithelium are unclear, but we propose that recapitulation of pathways and processes critical to embryological development associated with a tissue specific age-related stochastic epigenetic drift may be implicated. These pathways may also contribute to the distinctive behaviour of IPF fibroblasts. Genomic and epigenomic studies have revealed that wingless/ Int, sonic hedgehog and other developmental signalling pathways are reactivated and deregulated in IPF. Moreover, some of these pathways cross-talk with transforming growth factor-β activating a profibrotic feedback loop. The expression pattern of microRNAs is also dysregulated in IPF and exhibits a similar expression profile to embryonic lungs. In addition, senescence, a process usually associated with ageing, which occurs early in alveolar epithelial cells of IPF lungs, likely represents a conserved programmed developmental mechanism. -
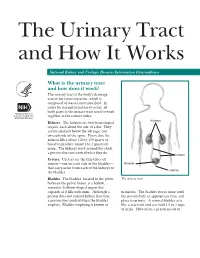
The Urinary Tract and How It Works
The Urinary Tract and How It Works National Kidney and Urologic Diseases Information Clearinghouse What is the urinary tract and how does it work? The urinary tract is the body’s drainage system for removing urine, which is composed of wastes and extra fluid. In order for normal urination to occur, all body parts in the urinary tract need to work together in the correct order. Kidneys Kidneys. The kidneys are two bean-shaped organs, each about the size of a fist. They are located just below the rib cage, one on each side of the spine. Every day, the kidneys filter about 120 to 150 quarts of blood to produce about 1 to 2 quarts of urine. The kidneys work around the clock; a person does not control what they do. Ureters Ureters. Ureters are the thin tubes of muscle—one on each side of the bladder— Bladder that carry urine from each of the kidneys to Urethra the bladder. Bladder. The bladder, located in the pelvis The urinary tract between the pelvic bones, is a hollow, muscular, balloon-shaped organ that expands as it fills with urine. Although a urination. The bladder stores urine until person does not control kidney function, the person finds an appropriate time and a person does control when the bladder place to urinate. A normal bladder acts empties. Bladder emptying is known as like a reservoir and can hold 1.5 to 2 cups of urine. How often a person needs to urinate depends on how quickly the kidneys Why is the urinary tract produce the urine that fills the bladder. -

Myofibroblasts Correlate with Lymphatic Microvessel Density and Lymph Node Metastasis in Early-Stage Invasive Colorectal Carcinoma
ANTICANCER RESEARCH 25: 2705-2712 (2005) Myofibroblasts Correlate with Lymphatic Microvessel Density and Lymph Node Metastasis in Early-stage Invasive Colorectal Carcinoma PIN LIANG1, JIAN-WEI HONG2, HIDEYUKI UBUKATA1, GE LIU1, MOTONOBU KATANO1, GYO MOTOHASHI1, TERUHIKO KASUGA1, YOSHINORI WATANABE1, ICHIRO NAKADA1 and TAKAFUMI TABUCHI1 1Fourth Department of Surgery and 2Department of Pathology, Tokyo Medical University Kasumigaura Hospital, Ibaraki, Japan Abstract. Background: Recent studies have shown that the Myofibroblasts are the main component cells in tumor interactions between tumor cells and stromal cells are stroma, and alpha-smooth muscle actin (·-SMA)-positive important in tumor development. A possible correlation myofibroblasts were found to participate in the synthesis of between tumor-activated myofibroblasts, the main component extracellular matrix components of tumor stroma, and to cells of tumor stroma, and lymphatic microvessel density produce lytic enzymes able to degrade the basement (LMVD) or other clinical parameters in carcinoma was membrane surrounding tumor glands. Although present in investigated. Materials and Methods: Immunohistochemical the progressive tumor nodules, they disappear during tumor examination of alpha-smooth muscle actin and podoplanin regression (5, 6). The correlation between microvessel were performed in 83 cases of early-stage invasive colorectal density and myofibroblasts was shown by Zidar et al. (7), but carcinoma. Results: There was a good correlation between the influence of myofibroblasts in lymphagiogenesis remains proliferation of myofibroblasts (PMpt) and LMVD (LMVDpt) unclear. Only recently, podoplanin, a 43-kd glomerular in the peri-tumoral area (p=0.0034). Increased PMpt was also podocyte membrane mucoprotein and a specific lymphatic associated with lymphatic invasion (p=0.0051) and with vessel marker, has enabled the investigation of the lymph node metastasis (p=0.011).