Signaling During Kidney Development
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Structure of Pronephros and Development of Mesonephric Kidney in Larvae of Russian Sturgeon, Acipenser Gueldenstaedtii Brandt (Acipenseridae)
Zoologica5 PRONEPHROS Poloniae-AND (2012)-MESONEPHRIC 57/1-4: 5-20-KIDNEY-IN-LARVAE-OF-A.-GUELDENSTAEDTII 5 DOI: 10.2478/v10049-012-0001-6 STRUCTURE OF PRONEPHROS AND DEVELOPMENT OF MESONEPHRIC KIDNEY IN LARVAE OF RUSSIAN STURGEON, ACIPENSER GUELDENSTAEDTII BRANDT (ACIPENSERIDAE) L.S. KRAYUSHKINA*1, A.A. GERASIMOV1, A.A. KIRSANOV1, M.V. MOSYAGINA1, A. OGORZA£EK2 1Department of Ichthyology and Hydrobiology, St. Petersburg State University, 16-th Line 29, 199178, St. Petersburg, Russia, [email protected] 2 Department of Animal Developmental Biology, Zoological Institute, University of Wroclaw, Sienkiewicza 21, 50-335 Wroclaw, Poland. *Corresponding author Abstract. The structure of the pronephros and development of mesonephric kidney in Russian sturgeon larvae, Acipenser gueldenstaedtii Brandt at different stages of early postembryonic development (from hatching to 14 days), were studied with histological and electronic microscopy methods. The larval pronephros is represented by the system of bilaterally located pronephric tubules with ciliated nephrostomes and funnels and exog- enous single glomus, which is not integrated directly into pronephric tubules and located in the pronephric chamber. The glomus is positioned below the dorsal aorta and vascular- ized by its capillaries. The glomus has the same features of the thin structure that are typical of and necessary for the function of a filtering organ. The structure of the prone- phros in acipenserids is discussed and compared with teleosts and amphibians. Histogen- esis of the mesonephric kidney is observed during the period of pronephros functioning; it is complete by the time the larvae transfer to exogenous feeding. At this moment, the pronephros undergoes significant structural degradation. -

Essential Roles of Inhibin Beta a in Mouse Epididymal Coiling
Essential roles of inhibin beta A in mouse epididymal coiling Jessica Tomaszewski*, Avenel Joseph†, Denise Archambeault†, and Humphrey Hung-Chang Yao†‡ *Department of Biology, School of Integrative Biology, and †Department of Veterinary Biosciences, College of Veterinary Medicine, University of Illinois at Urbana–Champaign, Urbana, IL 61802 Edited by Jean D. Wilson, University of Texas Southwestern Medical Center, Dallas, TX, and approved May 29, 2007 (received for review April 13, 2007) Testis-derived testosterone has been recognized as the key factor appear to be indirect via a mesenchyme-derived regulator(s). When for morphogenesis of the Wolffian duct, the precursor of several the upper Wolffian duct epithelium (the future epididymis) was male reproductive tract structures. Evidence supports that testos- grafted onto the lower Wolffian duct mesenchyme (the future terone is required for the maintenance of the Wolffian duct via its seminal vesicle), the epithelium underwent seminal vesicle mor- action on the mesenchyme. However, it remains uncertain how phogenesis and expressed markers specific for seminal vesicle testosterone alone is able to facilitate formation of regionally epithelium instead of those for epididymal epithelium (10). This specific structures such as the epididymis, vas deferens, and sem- inductive ability of Wolffian duct mesenchyme was also found in the inal vesicle from a straight Wolffian duct. In this study, we iden- prostate, providing further support that AR in the mesenchyme is tified inhibin beta A (or Inhba) as a regional paracrine factor in necessary to dictate androgenic actions of the epithelium (11). mouse mesonephroi that controls coiling of the epithelium in the Furthermore, when the epithelium from the AR-deficient testicular anterior Wolffian duct, the future epididymis. -

Kidney, Renal Tubule – Dilation
Kidney, Renal Tubule – Dilation Figure Legend: Figure 1 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Dilated tubules are noted as tracts running through the cortex and outer medulla. Figure 2 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is present throughout the outer stripe of the outer medulla, extending into the cortex. Figure 3 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Slight tubule dilation is associated with degeneration and necrosis. Figure 4 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is associated with chronic progressive nephropathy. Comment: Renal tubule dilation may occur anywhere along the nephron or collecting duct system. It may occur in focal areas or as tracts running along the entire length of kidney sections (Figure 1). 1 Kidney, Renal Tubule – Dilation Renal tubule dilation may occur from xenobiotic administration, secondary mechanisms, or an unknown pathogenesis (see Kidney – Nephropathy, Obstructive (Figure 2). Dilation may result from direct toxic injury to the tubule epithelium interfering with absorption and secretion (Figure 3). It may also occur secondary to renal ischemia or from prolonged diuresis related to drug administration. Secondary mechanisms of tubule dilation may result from lower urinary tract obstruction, the deposition of tubule crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy (Figure 4). A few dilated tubules may be regarded as normal histologic variation. Recommendation: Renal tubule dilation should be diagnosed and given a severity grade. The location of tubule dilation should be included in the diagnosis as a site modifier. -

Female Urethra
OBJECTIVES: • By the end of this lecture, student should understand the anatomical structure of urinary system. General Information Waste products of metabolism are toxic (CO2, ammonia, etc.) Removal from tissues by blood and lymph Removal from blood by Respiratory system And Urinary system Functions of the Urinary System Elimination of waste products Nitrogenous wastes Toxins Drugs Functions of the Urinary System Regulate homeostasis Water balance Acid-base balance in the blood Electrolytes Blood pressure Organs of the Urinary system Kidneys Ureters Urinary bladder Urethra Kidneys Primary organs of the urinary system Located between the 12th thoracic and 3rd lumbar vertebrae. Right is usually lower due to liver. Held in place by connective tissue [renal fascia] and surrounded by thick layer of adipose [perirenal fat] Each kidney is approx. 3 cm thick, 6 cm wide and 12 cm long Regions of the Kidney Renal cortex: outer region Renal medulla: pyramids and columns Renal pelvis: collecting system Kidneys protected by three connective tissue layers Renal fascia -Attaches to abdominal wall Renal capsule: -Surrounds each kidney -Fibrous sac -Protects from trauma and infection Adipose capsule -Fat cushioning kidney Nephrons Each kidney contains over a million nephrons [functional structure] • Blood enters the nephron from a network that begins with the renal artery. • This artery branches into smaller and smaller vessels and enters each nephron as an afferent arteriole. • The afferent arteriole ends in a specialized capillary called the Glomerulus. • Each kidney has a glomerulus contained in Bowman’s Capsule. • Any cells that are too large to pass into the nephron are returned to the venous blood supply via the efferent arteriole. -

Stem Cells in the Embryonic Kidney R Nishinakamura1
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector http://www.kidney-international.org mini review & 2008 International Society of Nephrology Stem cells in the embryonic kidney R Nishinakamura1 1Division of Integrative Cell Biology, Institute of Molecular Embryology and Genetics, Kumamoto University, 2-2-1 Honjo, Kumamoto, Japan The mammalian kidney, the metanephros, is formed by a STRATEGY TOWARD KIDNEY RECONSTITUTION USING reciprocally inductive interaction between two precursor PROGENITOR CELLS tissues, the metanephric mesenchyme and the ureteric bud. Stem cells are defined by two criteria: self-renewal and The ureteric bud induces the metanephric mesenchyme to multipotency. Few reports in the kidney field have addressed differentiate into the epithelia of glomeruli and renal tubules. both of these criteria at a clonal level, so it is better to use the Multipotent renal progenitors that form colonies upon Wnt4 term ‘progenitor’ rather than ‘stem cells.’ In this review, renal stimulation and strongly express Sall1 exist in the progenitors in the embryonic kidney, not those in the adult metanephric mesenchyme; these cells can partially kidney, from the viewpoint of developmental biology and reconstitute a three-dimensional structure in an organ stem/progenitor cell biology will be discussed. To generate culture setting. Six2 maintains this mesenchymal progenitor multiple cell lineages for kidney regeneration, the identifica- population by opposing Wnt4-mediated epithelialization. tion of renal progenitors is a prerequisite. Furthermore, there Upon epithelial tube formation, Notch2 is required for the exist three obstacles to be overcome: (1) derivation of the differentiation of proximal nephron structures (podocyte and renal progenitors; (2) expansion of the renal progenitors; and proximal tubules). -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -
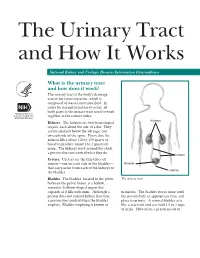
The Urinary Tract and How It Works
The Urinary Tract and How It Works National Kidney and Urologic Diseases Information Clearinghouse What is the urinary tract and how does it work? The urinary tract is the body’s drainage system for removing urine, which is composed of wastes and extra fluid. In order for normal urination to occur, all body parts in the urinary tract need to work together in the correct order. Kidneys Kidneys. The kidneys are two bean-shaped organs, each about the size of a fist. They are located just below the rib cage, one on each side of the spine. Every day, the kidneys filter about 120 to 150 quarts of blood to produce about 1 to 2 quarts of urine. The kidneys work around the clock; a person does not control what they do. Ureters Ureters. Ureters are the thin tubes of muscle—one on each side of the bladder— Bladder that carry urine from each of the kidneys to Urethra the bladder. Bladder. The bladder, located in the pelvis The urinary tract between the pelvic bones, is a hollow, muscular, balloon-shaped organ that expands as it fills with urine. Although a urination. The bladder stores urine until person does not control kidney function, the person finds an appropriate time and a person does control when the bladder place to urinate. A normal bladder acts empties. Bladder emptying is known as like a reservoir and can hold 1.5 to 2 cups of urine. How often a person needs to urinate depends on how quickly the kidneys Why is the urinary tract produce the urine that fills the bladder. -

Secreted Molecules in Metanephric Induction
J Am Soc Nephrol 11: S116–S119, 2000 Secreted Molecules in Metanephric Induction THOMAS J. CARROLL and ANDREW P. McMAHON Department of Molecular and Cellular Biology, Biological Laboratories, Harvard University, Cambridge, Massachusetts. Abstract. Nearly 50 yr ago, Clifford Grobstein made the ob- the classic model of metanephric induction. The studies of the servation that the ureteric bud induced the nephrogenic mes- classic ureteric inducer performed to date have most likely enchyme to undergo tubulogenesis. Since that discovery, sci- been characterizations of a mesenchyme-specific inducer, entists have attempted to characterize the molecular nature of Wnt-4, and its role in tubulogenesis. Ureteric induction most the inducer. To date, no single molecule that is both necessary likely involves a series of distinct events that provide prolif- and sufficient for nephric induction has been identified. Be- erative, survival, and condensation signals to the mesenchyme, cause of recent insights regarding the role of several secreted integrating the growth of the ureteric system with tubulogen- molecules in tubulogenesis, it has become necessary to revise esis. The developmental biologic processes of the kidney have been logenesis. The conclusion drawn from this discovery was that the subject of intense study for more than 100 yr (for review, the ureteric bud induces tubulogenesis within the surrounding see reference (1). All three vertebrate kidney types (pro- mesenchyme. During further investigation, it was discovered nephros, mesonephros, and metanephros) are derivatives of a that a number of tissues, including, most notably, a dorsal region of the embryo known as the intermediate mesoderm. In portion of the embryonic spinal cord, are able to substitute for mice, a portion of the mesonephric duct, known as the meta- the ureter in this inductive interaction. -

Claudins in the Renal Collecting Duct
International Journal of Molecular Sciences Review Claudins in the Renal Collecting Duct Janna Leiz 1,2 and Kai M. Schmidt-Ott 1,2,3,* 1 Department of Nephrology and Intensive Care Medicine, Charité-Universitätsmedizin Berlin, 12203 Berlin, Germany; [email protected] 2 Molecular and Translational Kidney Research, Max-Delbrück-Center for Molecular Medicine in the Helmholtz Association (MDC), 13125 Berlin, Germany 3 Berlin Institute of Health (BIH), 10178 Berlin, Germany * Correspondence: [email protected]; Tel.: +49-(0)30-450614671 Received: 22 October 2019; Accepted: 20 December 2019; Published: 28 December 2019 Abstract: The renal collecting duct fine-tunes urinary composition, and thereby, coordinates key physiological processes, such as volume/blood pressure regulation, electrolyte-free water reabsorption, and acid-base homeostasis. The collecting duct epithelium is comprised of a tight epithelial barrier resulting in a strict separation of intraluminal urine and the interstitium. Tight junctions are key players in enforcing this barrier and in regulating paracellular transport of solutes across the epithelium. The features of tight junctions across different epithelia are strongly determined by their molecular composition. Claudins are particularly important structural components of tight junctions because they confer barrier and transport properties. In the collecting duct, a specific set of claudins (Cldn-3, Cldn-4, Cldn-7, Cldn-8) is expressed, and each of these claudins has been implicated in mediating aspects of the specific properties of its tight junction. The functional disruption of individual claudins or of the overall barrier function results in defects of blood pressure and water homeostasis. In this concise review, we provide an overview of the current knowledge on the role of the collecting duct epithelial barrier and of claudins in collecting duct function and pathophysiology. -

Glomus Specification and Induction in Xenopus
Development 126, 5847-5856 (1999) 5847 Printed in Great Britain © The Company of Biologists Limited 1999 DEV6378 The specification and growth factor inducibility of the pronephric glomus in Xenopus laevis Hannah C. Brennan, Sarbjit Nijjar and Elizabeth A. Jones* Cell and Molecular Development Group, Department of Biological Sciences, Warwick University, Coventry, CV4 7AL, UK *Author for correspondence (e-mail: [email protected]) Accepted 23 September; published on WWW 24 November 1999 SUMMARY We report a study on the specification of the glomus, the Furthermore, we have analysed the growth factor filtration device of the amphibian pronephric kidney, using inducibility of the glomus in the presence or absence of an explant culturing strategy in Xenopus laevis. Explants of retinoic acid (RA) by RT-PCR. We define for the first time presumptive pronephric mesoderm were dissected from the conditions under which these growth factors induce embryos of mid-gastrula to swimming tadpole stages. These glomus tissue in animal cap tissue. Activin together with explants were cultured within ectodermal wraps and high concentrations of RA can induce glomus tissue from analysed by RT-PCR for the presence of the Wilm’s Tumour- animal cap ectoderm. Unlike the pronephric tubules, the 1 gene, xWT1, a marker specific for the glomus at the stages glomus can also be induced by FGF and RA. analysed, together with other mesodermal markers. We show that the glomus is specified at stage 12.5, the same stage at which pronephric tubules are specified. We have previously shown that pronephric duct is specified somewhat later, at stage 14. -

ROBO2 Restricts the Nephrogenic Field and Regulates Wolffian Duct–Nephrogenic Cord Separation
Developmental Biology 404 (2015) 88–102 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/locate/developmentalbiology ROBO2 restricts the nephrogenic field and regulates Wolffian duct–nephrogenic cord separation Elanor N. Wainwright 1, Dagmar Wilhelm 2, Alexander N. Combes 3, Melissa H. Little 4, Peter Koopman n Institute for Molecular Bioscience, The University of Queensland, Brisbane, QLD 4072, Australia article info abstract Article history: ROBO2 plays a key role in regulating ureteric bud (UB) formation in the embryo, with mutations in Received 7 April 2015 humans and mice leading to supernumerary kidneys. Previous studies have established that the number Received in revised form and position of UB outgrowths is determined by the domain of metanephric mesenchymal Gdnf ex- 28 May 2015 pression, which is expanded anteriorly in Robo2 mouse mutants. To clarify how this phenotype arises, we Accepted 30 May 2015 used high-resolution 3D imaging to reveal an increase in the number of nephrogenic cord cells, leading Available online 23 June 2015 to extension of the metanephric mesenchyme field in Robo2-null mouse embryos. Ex vivo experiments Keywords: suggested a dependence of this effect on proliferative signals from the Wolffian duct. Loss of Robo2 Kidney resulted in a failure of the normal separation of the mesenchyme from the Wolffian duct/ureteric epi- Wolffian duct thelium, suggesting that aberrant juxtaposition of these two compartments in Robo2-null mice exposes Ureteric bud the mesenchyme to abnormally high levels of proliferative stimuli. Our data suggest a new model in Nephrogenic cord Mouse which SLIT-ROBO signalling acts not by attenuating Gdnf expression or activity, but instead by limiting epithelial/mesenchymal interactions in the nascent metanephros and restricting the extent of the ne- phrogenic field.