Tools to Qualify Experiments with Bloomery Furnaces*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
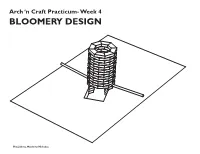
Bloomery Design
Arch ‘n Craft Practicum- Week 4 BLOOMERY DESIGN Hiu, Julieta, Matthew, Nicholas 7 3/4" The Stack: 3 3/4" In our ideal model we would use refractory bricks, either rectangular bricks or curved bricks which would help us make a circular 1'-8" stack. The stack would be around 3 feet tall with a 1 foot diameter for the inner wall of the stack. There will be openings about 3 brick lengths up(6-9 inches) from the ground on 1'-0" opposite ends of the stack. Attached to these openings will be our tuyeres which in turn will connect to our air sources. Building a circular stack would be beneficial to the type of air distribution we want to create within our furnace; using two angled tuyeres we would like air to move clockwise and counterclock- Bloomery Plan wise. We would like to line the inside of the stack with clay to provide further insulation. Refractory bricks would be an ideal material to use because the minerals they are com- posed of do not change chemically when 1'-0" exposed to high heat, some examples of these materials are aluminum, manganese, silica and zirconium. Other comments: 3'-0" Although we have opted to use refractory bricks, which would be practical for our intended purpose, we briefly considered using Vycor glass which can withstand high tem- peratures of up to 900 degrees celsius or approximately 1652 degrees Fahrenheit, which Tuyere height: Pitched -10from horizontal 7" would be enough for our purposes. Bloomery Section Ore/ Charcoal: Our group has decided to use Magnetite ore along with charcoal for the smelt. -

From Bloomery Furnace to Blast Furnace Archeometallurgical Analysis of Medieval Iron Objects from Sigtuna and Lapphyttan, Sverige
EXAMENSARBETE INOM TEKNIK, GRUNDNIVÅ, 15 HP STOCKHOLM, SVERIGE 2019 From Bloomery Furnace to Blast Furnace Archeometallurgical Analysis of Medieval Iron Objects From Sigtuna and Lapphyttan, Sverige ANDREAS HELÉN ANDREAS PETTERSSON KTH SKOLAN FÖR INDUSTRIELL TEKNIK OCH MANAGEMENT Abstract During the Early Middle Ages, the iron production in Sweden depended on the bloomery furnace, which up to that point was well established as the only way to produce iron. Around the Late Middle Ages, the blast furnace was introduced in Sweden. This made it possible to melt the iron, allowing it to obtain a higher carbon composition and thereby form new iron-carbon phases. This study examines the microstructure and hardness of several tools and objects originating from archaeological excavations of Medieval Sigtuna and Lapphyttan. The aim is to examine the differences in quality and material properties of iron produced by respectively blast furnaces and bloomery furnaces. Both methods required post-processing of the produced iron, i.e. decarburization for blast furnaces and carburization for bloomeries. These processes were also studied, to better understand why and how the material properties and qualities of the items may differ. The results show that some of the studied items must have been produced from blast furnace iron, due to their material composition and structure. These items showed overall better material quality and contained less slag. This was concluded because of the increased carbon concentration that allowed harder and more durable structures such as pearlite to form. The study also involved an investigation of medieval scissors, also known as shears, made from carburized bloomery furnace iron. -

Bloomery (Edited from Wikipedia)
Bloomery (Edited from Wikipedia) SUMMARY A bloomery is a type of furnace once widely used for smelting iron from its oxides. The bloomery was the earliest form of smelter capable of smelting iron. The result of using a bloomery is a mass of iron and slag called a bloom. This is called sponge iron, because it is porous like a sponge. Sponge iron is usually refined into wrought iron. Bloomeries are no longer used in smelting, because blast furnaces are better. HISTORY China China has long been considered the exception to the general use of bloomeries. It is thought that the Chinese skipped the bloomery process completely, starting with the blast furnace and the finery forge to get wrought iron: by the 5th century BC, metalworkers had invented the blast furnace. Sub-Saharan Africa Smelting in bloomery type furnaces in West Africa and forging of tools appeared by 500 BC. Medieval Europe Early European bloomeries were relatively small, smelting less than 1 kg of iron with each firing. Progressively larger bloomeries were constructed in the late fourteenth century, with a capacity of about 15 kg on average, though exceptions did exist. The use of waterwheels to power the bellows allowed the bloomery to become larger and hotter. European average bloom sizes quickly rose to 300 kg, where they leveled off until the demise of the bloomery. As a bloomery's size is increased, the iron ore is exposed to burning charcoal for a longer time. When combined with the strong air blast required to penetrate the large ore and charcoal stack, this may cause part of the iron to melt and become saturated with carbon in the process, producing unforgeable pig iron which requires oxidation to be reduced into cast iron, steel, and iron. -

The Production of High Carbon Steel Directly in Bloomery Process: Theoretical Bases and Metallographic Analyses of the Experiments Results
EXARC JOURNAL, ISSUE 2013/2 The Production of High Carbon Steel Directly in Bloomery Process: Theoretical Bases and Metallographic Analyses of the Experiments Results Adrian Wrona (PL) The problem of steel1 making in antiquity has intrigued researchers who specialize in ancient metallurgy for decades. In the course of research different explanations have prevailed in the scholarship. R. J. Forbes (1950, 405-412) in his classic work Metallurgy in Antiquity distinguished three main methods of steel production: 1. Steel obtained directly in the bloomery process. However, he points out that high carbon steel was only a small part of smelted bloom and was not able to fulfil the population's needs for a high-quality material used to produce the necessary tools. He argued his thesis by poor ores used by ancient smelters, low temperature of process, et cetera. 2. As a second method, Forbes mentions the possibility of decarburization of cast iron obtained during the smelting process, but he rejects the intentional receive of it in the reduction process. The fragments of pig iron occasionally found, mostly on the Roman metallurgical sites, are (according to Forbes) accidental by-products that have been abandoned as an useless and not suitable for further processing. 3. A third possibility is making steel by carburization of soft iron, commonly called cementation or casehardening. This distinction came to form the basis our knowledge on the subject, and these methods became a basis for further studies and explanations about carburizing technique used in tool and weapon production. For a long time the third possibility was considered the main method for the preparation . -

Mechanical Properties of Medieval Bloomery Iron Materials 2015 59 1 35 Table 1 Samples for Mechanical Testing
PP Periodica Polytechnica Mechanical Properties of Medieval Mechanical Engineering Bloomery Iron Materials - Comparative Tensile and Charpy-tests on Bloomery 59(1), pp. 35-38, 2015 Iron Samples and S235JRG2 DOI: 10.3311/PPme.7760 Creative Commons Attribution b Ádám Thiele1 *, Jiří Hošek2 research article Received 16 October 2014; accepted after revision 10 November 2014 Abstract 1 Introduction Ductility, toughness and strength of medieval bloomery iron Based on numerous investigations carried out on both materials were highly important mechanical properties, archaeological and experimentally yielded bloomery iron arte- strongly affected by their microstructure and chemical compo- facts, it can be stated that the microstructure and the chemical sition. An attempt was made to characterize the most impor- composition of bloomery irons and steels differ from those of tant mechanical properties of representative samples of main modern steels. Since microstructure and chemical composition bloomery iron materials extracted in smelting experiments as well as purity strongly affect mechanical properties, these and compare them to the well know reference modern steel of must differ from those of modern steels as well. Therefore, S235JRG2. It was confirmed that notching and the stress con- even for well-trained material engineers it might have been dif- centration effect of slag inclusions strongly decrease all the ficult to reliably assess mechanical properties (mainly tough- characteristic values of ductility and toughness of bloomery ness and strength) of such historic objects as swords, which in iron materials. Typical medieval bloomery P-iron is a brittle addition often reveal high complexity in terms of materials and material with almost zero or very low characteristic values of their various combinations used within the manufacture of a ductility and toughness but revealed similarly high strength as single object. -

Iron, Steel and Swords Script - Page 1 the Basic Technique Is Exactly the Same in Both Cases: Take a Rock and Chisel Off What You Don't Want
10. 5. Iron and Steel in "Modern" Europe 10.5.1 From Bloomeries to the Blast Furnace Changes Over Time - a General View In the preceding subchapters I covered the early history of iron and steel in some detail: from the beginning to about Roman times in Europe, or up to - roughly - 400 AD. Other parts of the world were mostly neglected with the exception of a short entry for China, and a not-so-short subchapter about crucible steel in India and parts of Asia. There was no iron and steel in the Americas and Australia before the "white man" brought it along, so there is nothing to tell. Africa, however, does have a "modern" iron and steel history; I deal with it - shortly - here. What follows is heavily biased towards (Northern) Europe. So what has happened with respect to iron an steel development between - roughly - 400 AD and 1870 AD in Europe? Looking at one of the time lines I have given you before for smelting, one is inclined to conclude: not much! Bloomeries were still run in the 17th century or even later, and the basic process was not much different from the one used 1500 years earlier. A sword was still forged by a smith. A 200 BC Celt coming out of some deep freeze in 1500 would hardly note a difference in the forging process. So the basic techniques for making swords and other iron / steel items didn't change much. That is also quite typical for other cultural / technical developments. Does that mean that the products didn't change much either? Just for fun, let's look at how the changes of products relate to the changes or non-changes in basic technologies. -

Steelmaking: History and the Modern Age
October 14, 2020 Steelmaking: History and the Modern Age What is necessary to cook a dish? Temperature. A few centuries ago, its source was an open fire of wood and coal, while today's kitchens are equipped with gas or electric ovens. Steel-smelting in the metallurgical kitchen is performed in a similar fashion: raw materials (the charge) are added into a huge "pot" and "boiled" at high temperatures according to a certain kind of technology (the recipe). At the same time, the right temperature is achieved by the use of gas or electricity. At the moment, there are three primary, industrial ways of smelting steel in the world: the OHF process; the BOF process; the electro-metallurgical process. History of steel smelting Humankind learned to make iron in the Middle Ages. However, up to the middle of the 19th century, it was in small amounts of low-quality materials. As a rule, it was produced in bloomery furnaces and refined in forges, where the makers obtained piece goods. Remarkably, remains of medieval bloomery furnaces (also known as hammer workshops) have been found on the territory of modern Ukraine. Most notably, they were located in the western part of the country, which is not a centre of the steel industry today. The technologies used before the 19th century to manufacture iron products had one significant drawback: either very soft iron or fragile steel made of iron was produced in the forges. Such materials could not be used in their pure state, as the products quickly dulled or could be easily broken. -

The Carp River Bloomery Iron Forge
“... A Monument to Misguided Enterprise”: The Carp River Bloomery Iron Forge David Landon, Patrick Martin, Andrew Sewell, Paul White, Timothy Tumberg, and Jason Menard The mid-19th-century Carp River Forge was the first iron ment, tempered by sweat and optimism. Established by the smelting operation on the Marquette Iron Range, launching Jackson Iron Company in 1847, the forge was the first site the iron industry of Michigan’s Upper Peninsula. At the forge, of iron production in the Upper Peninsula (UP), proving skilled ironworkers produced iron in bloomery hearths using the value of the district’s rich hematite ores and opening charcoal, ore, and a water-powered air blast. This paper pre- Michigan’s Marquette Iron Range.1 Over the next 10 years, sents the results of the historical research and three seasons of other iron companies followed the Jackson Company’s excavation at the site. Major archaeological discoveries lead, constructing bloomery forges at three other locations include the dam base, the water wheel gudgeon and crank, in the region (Figure 1).2 All of these forges produced iron parts of the bloomery forges, a blacksmith’s forge base, and using a direct-reduction process to make wrought iron the remains of houses for the forge workers. The archaeologi- from ore; three of the forges, including the Carp River cal remains of the bloomery forges suggest the forge workers Forge, used water power to drive machinery. All four of the employed the latest hot-air blast and firebox design. The spa- bloomery ironworks had short lives, going out of operation tial distribution of the ore, charcoal, and waste slag, in con- by the end of the 1850s with an estimated total output of junction with the industrial features, defines the layout and less than 15,000 tons of iron.3 None of the forges ever organization of the industrial workings. -

The Basics of Bloomery Smelting
THE BASICS OF BLOOMERY SMELTING Deep in my January reading binge of 1998, inspired by accounts of African iron smelting and an account of the 19th century iron industry in my home county, I hatched the notion to smelt my own iron from local ore, and make a sculpture with it. Until I read about the African bloomery furnace, I had never considered the possibility of iron being smelted on a small scale. It was one of those deadly ideas that once surfaced, would not leave, so I enlisted the help of my fellow eccentric Skip Williams. We thought a little ironmaking would be a fun way to spend a few chilly weekends. Fourteen months later, after many attempts and building two furnaces, we finally succeeded in smelting a 42-lb chunk of iron. So what I’m going to do here is try to tell you how I made it work, trying mightily not to spin too deeply into Digression Land. But first I must warn you here: one lesson I learned in this project was not to put too much stock in what I have read. So if you’re crazy enough to try this don’t blindly trust what anyone tells you. Trust your eyes and your experience and your instinct. WHAT IS A BLOOMERY? For those who don’t know, the bloomery process (also referred to as direct reduction) is the original method of producing iron. Operating on a small scale and at relatively low temperatures, it produced a sponge of malleable iron and slag that was forged directly into a wrought iron bar or billet. -

Cl1apter 4 the Ironworks Study
Cl1apter 4 _ The Ironworks Study sruc1~, Me1hoc1O1ogy ____ ________ were in use in others~ both types were fou nd at some of the Ironworks on an indus1rial scale co,mnenced in Vennon1 a few works (Jacobs 1953:130). Jacobs' source of infonnation was years after the end of the Revolutionary War and ended about a a rnanuscript sent to him by Charles Rufus Harce of New Haven. century later. During that JOO.year pei-iod. bias, furnaces, bloom Connecticut. In an April 5, 1955, letter 10 Richard S. Allen of e.ry forges, and foundries made or work{.-d iron in all or lhc state's Albany, New York. Marte wrote that what he sent Jacobs was 14 counties. A majority of the blast fu rnaces and bloomer)' forges "a copy of a series of abstracL~ from various Vermont town lay wesc of the Green Moumain range. generally in the valley of histories, and fo r their accuracy- <>ther than to the copying- ( che Ouer Creek. accepc no responsibility, and of che furnaces I have very little Because few business records of these industries remain, it is personal knowledge." not known for sure where all that iron went. Some Vermont iron Harte was otherwise an accurate and thorough historian of found markets in local foundries and mills. but most probably ironworks in New England, and authored a number of valuable ended up in works at Troy, New York, and Boston. Some iron a,'licles and booklets on the subject in the 1930s and 1940s. might even have found its way to markets in Quebec. -

Practical Bloomery Smelting W. H. Lee Sauder and Henry G. Williams III
Practical Bloomery Smelting W. H. Lee Sauder and Henry G. Williams III Woods Creek Forge, 229B McLaughlin Street, Lexington, VA 24450 Washington & Lee University, Lexington, VA 24450 ABSTRACT The art and technique of bloomery smelting, man’s original method for winning metallic iron from its ore, has been largely lost in recent centuries. Many reconstructions of this technique have been attempted by archaeologists in the last 30 years. These experimental smelts have tended to be rather disappointing in terms of the production of usable iron; nonetheless, many conclusions have been drawn from this work. The main goal of our work with bloomery smelting is the production of iron for the creation of forged sculpture. Our focus on producing a usable product has led us to a somewhat different view of the technology from what has been published in the archaeological literature. In this paper, we will briefly summarize our work through the spring of 2000, which has been published elsewhere. We’ll then report our recent findings from the 2000-2001 smelting season, describing a typical smelt of the most efficient smelting regimen we have yet discovered. We will pay particular attention to methods that differ from those of other experimenters, especially in regards to blowing rate, slag management, and the recycling of furnace products. Finally, we’ll point toward areas of upcoming research. INTRODUCTION Summary of early work We have been experimenting with the bloomery process since January of 1998. From the beginning, our primary goal has been to smelt iron of sufficient quantity and quality for the creation of hand-forged artworks, and to explore the process for a deeper understanding of iron as an artistic medium. -

Industrial Process Industrial Process
INDUSTRIAL PROCESS INDUSTRIAL PROCESS SESSION 9 IRON AND STEEL SESSION 9 IRON AND STEEL Session 9 Iron and steel 9 Iron and steel Early production of iron was from meteorites, or as a by-product of copper refining. Heating iron ore and carbon in a crucible at 1000 K produces wrought iron. This process gained popularity during the Iron Age. Temperatures of 1300 K were produced around the 8th century by blowing air through the heated mixture in a bloomery or blast furnace (12th century); producing a strong but brittle cast iron. Furnaces were growing bigger, producing greater quantities; a factor contributing to the Industrial Revolution. In 1740 the temperature and carbon content could be controlled sufficiently to consistently produce steel; very strong and very workable. The 19th century saw the development of electric arc furnaces that produced steel in very large quantities, and are more easily controlled. Smelting - the generic process used in furnaces to produce steel, copper, etc. Catalan forge, Open hearth furnace, Bloomery, Siemens regenerative furnace - produced wrought iron Blast furnace - produced cast iron Direct Reduction - produced direct reduced iron Crucible steel Cementation process Bessemer process Basic oxygen steelmaking, Linz-Donawitz process Electric arc furnace Smelting 1 Electric phosphate smelting furnace in a TVA chemical plant (1942) Smelting is a form of extractive metallurgy; its main use is to produce a metal from its ore. This includes production of silver, iron, copper and other base metals from their ores. Smelting uses heat and a chemical reducing agent to decompose the ore, driving off other elements as gasses or slag and leaving just the metal behind.