Bloomery Iron Smelting in the Daye County
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Landscape Analysis of Geographical Names in Hubei Province, China
Entropy 2014, 16, 6313-6337; doi:10.3390/e16126313 OPEN ACCESS entropy ISSN 1099-4300 www.mdpi.com/journal/entropy Article Landscape Analysis of Geographical Names in Hubei Province, China Xixi Chen 1, Tao Hu 1, Fu Ren 1,2,*, Deng Chen 1, Lan Li 1 and Nan Gao 1 1 School of Resource and Environment Science, Wuhan University, Luoyu Road 129, Wuhan 430079, China; E-Mails: [email protected] (X.C.); [email protected] (T.H.); [email protected] (D.C.); [email protected] (L.L.); [email protected] (N.G.) 2 Key Laboratory of Geographical Information System, Ministry of Education, Wuhan University, Luoyu Road 129, Wuhan 430079, China * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel: +86-27-87664557; Fax: +86-27-68778893. External Editor: Hwa-Lung Yu Received: 20 July 2014; in revised form: 31 October 2014 / Accepted: 26 November 2014 / Published: 1 December 2014 Abstract: Hubei Province is the hub of communications in central China, which directly determines its strategic position in the country’s development. Additionally, Hubei Province is well-known for its diverse landforms, including mountains, hills, mounds and plains. This area is called “The Province of Thousand Lakes” due to the abundance of water resources. Geographical names are exclusive names given to physical or anthropogenic geographic entities at specific spatial locations and are important signs by which humans understand natural and human activities. In this study, geographic information systems (GIS) technology is adopted to establish a geodatabase of geographical names with particular characteristics in Hubei Province and extract certain geomorphologic and environmental factors. -

Download Article
Advances in Economics, Business and Management Research, volume 70 International Conference on Economy, Management and Entrepreneurship(ICOEME 2018) Research on the Path of Deep Fusion and Integration Development of Wuhan and Ezhou Lijiang Zhao Chengxiu Teng School of Public Administration School of Public Administration Zhongnan University of Economics and Law Zhongnan University of Economics and Law Wuhan, China 430073 Wuhan, China 430073 Abstract—The integration development of Wuhan and urban integration of Wuhan and Hubei, rely on and Ezhou is a strategic task in Hubei Province. It is of great undertake Wuhan. Ezhou City takes the initiative to revise significance to enhance the primacy of provincial capital, form the overall urban and rural plan. Ezhou’s transportation a new pattern of productivity allocation, drive the development infrastructure is connected to the traffic artery of Wuhan in of provincial economy and upgrade the competitiveness of an all-around and three-dimensional way. At present, there provincial-level administrative regions. This paper discusses are 3 interconnected expressways including Shanghai- the path of deep integration development of Wuhan and Ezhou Chengdu expressway, Wuhan-Ezhou expressway and from the aspects of history, geography, politics and economy, Wugang expressway. In terms of market access, Wuhan East and puts forward some suggestions on relevant management Lake Development Zone and Ezhou Gedian Development principles and policies. Zone try out market access cooperation, and enterprises Keywords—urban regional cooperation; integration registered in Ezhou can be named with “Wuhan”. development; path III. THE SPACE FOR IMPROVEMENT IN THE INTEGRATION I. INTRODUCTION DEVELOPMENT OF WUHAN AND EZHOU Exploring the path of leapfrog development in inland The degree of integration development of Wuhan and areas is a common issue for the vast areas (that is to say, 500 Ezhou is lower than that of central urban area of Wuhan, and kilometers from the coastline) of China’s hinterland. -
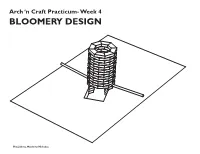
Bloomery Design
Arch ‘n Craft Practicum- Week 4 BLOOMERY DESIGN Hiu, Julieta, Matthew, Nicholas 7 3/4" The Stack: 3 3/4" In our ideal model we would use refractory bricks, either rectangular bricks or curved bricks which would help us make a circular 1'-8" stack. The stack would be around 3 feet tall with a 1 foot diameter for the inner wall of the stack. There will be openings about 3 brick lengths up(6-9 inches) from the ground on 1'-0" opposite ends of the stack. Attached to these openings will be our tuyeres which in turn will connect to our air sources. Building a circular stack would be beneficial to the type of air distribution we want to create within our furnace; using two angled tuyeres we would like air to move clockwise and counterclock- Bloomery Plan wise. We would like to line the inside of the stack with clay to provide further insulation. Refractory bricks would be an ideal material to use because the minerals they are com- posed of do not change chemically when 1'-0" exposed to high heat, some examples of these materials are aluminum, manganese, silica and zirconium. Other comments: 3'-0" Although we have opted to use refractory bricks, which would be practical for our intended purpose, we briefly considered using Vycor glass which can withstand high tem- peratures of up to 900 degrees celsius or approximately 1652 degrees Fahrenheit, which Tuyere height: Pitched -10from horizontal 7" would be enough for our purposes. Bloomery Section Ore/ Charcoal: Our group has decided to use Magnetite ore along with charcoal for the smelt. -

Download Article
Advances in Social Science, Education and Humanities Research, volume 195 International Seminar on Education Research and Social Science (ISERSS 18) Research on the Rural Homestay in Xiangyang City Jia Huijun Xiangyang Vocational and Technical College Xiangyang, Hubei, 441021 Abstract—With the development of the economy and the kind is that the word is from the Minshuku of Japan, which is improvement of living standards, tourists have diversified derived and developed by some people who love climbing pursuit of travel services and products. For example, there are mountains, skiing and swimming renting the local houses; and theme hotels, vacationing hotels, Traders Hotel, and homestay the other is that homestays are emerged in Europe and the US, for tourists’ staying. The homestay has its own unique represented by British B&B and American Home stay. As far characteristics and development. We mainly analysis and look as China is concerned, the first one is relatively reasonable. into the future of the development of homestay in villages in Although it cannot be accurately verified from all over the Xiangyang City through the analysis of the status of the world, the shadow of Japanese homestay can be clearly seen development of the hotel in China and the development of Hube. from the development of China’s Taiwan region. In China, We should learn from the surrounding provinces and cities, Taiwan was the earliest area to develop homestay. In the early improve the full meaning, seize the opportunities for the 1980s, Kenting national park in Taiwan derived a kind of development of the new era, and then the people can achieve higher economic benefits and lead the development of tourism. -

Milankovitch and Sub-Milankovitch Cycles of the Early Triassic Daye Formation, South China and Their Geochronological and Paleoclimatic Implications
Gondwana Research 22 (2012) 748–759 Contents lists available at SciVerse ScienceDirect Gondwana Research journal homepage: www.elsevier.com/locate/gr Milankovitch and sub-Milankovitch cycles of the early Triassic Daye Formation, South China and their geochronological and paleoclimatic implications Huaichun Wu a,b,⁎, Shihong Zhang a, Qinglai Feng c, Ganqing Jiang d, Haiyan Li a, Tianshui Yang a a State Key Laboratory of Geobiology and Environmental Geology, China University of Geosciences, Beijing 100083, China b School of Ocean Sciences, China University of Geosciences (Beijing), Beijing 100083 , China c State Key Laboratory of Geological Processes and Mineral Resources, China University of Geosciences, Wuhan 430074, China d Department of Geoscience, University of Nevada, Las Vegas, NV 89154, USA article info abstract Article history: The mass extinction at the end of Permian was followed by a prolonged recovery process with multiple Received 16 June 2011 phases of devastation–restoration of marine ecosystems in Early Triassic. The time framework for the Early Received in revised form 25 November 2011 Triassic geological, biological and geochemical events is traditionally established by conodont biostratigra- Accepted 2 December 2011 phy, but the absolute duration of conodont biozones are not well constrained. In this study, a rock magnetic Available online 16 December 2011 cyclostratigraphy, based on high-resolution analysis (2440 samples) of magnetic susceptibility (MS) and Handling Editor: J.G. Meert anhysteretic remanent magnetization (ARM) intensity variations, was developed for the 55.1-m-thick, Early Triassic Lower Daye Formation at the Daxiakou section, Hubei province in South China. The Lower Keywords: Daye Formation shows exceptionally well-preserved lithological cycles with alternating thinly-bedded mud- Early Triassic stone, marls and limestone, which are closely tracked by the MS and ARM variations. -

Climate Research 50:161
Vol. 50: 161–170, 2011 CLIMATE RESEARCH Published December 22 doi: 10.3354/cr01052 Clim Res Contribution to CR Special 28 ‘Changes in climatic extremes over mainland China’ OPENPEN ACCESSCCESS Historical analogues of the 2008 extreme snow event over Central and Southern China Zhixin Hao1, Jingyun Zheng1, Quansheng Ge1,*, Wei-Chyung Wang2 1Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, 11A Datun Road, Chaoyang District, Beijing 100101, PR China 2Atmospheric Sciences Research Center, State University of New York, Albany, New York 12203, USA ABSTRACT: We used weather records contained in Chinese historical documents from the past 500 yr to search for extreme snow events (ESEs) that were comparable in severity to an event in early 2008, when Central and Southern China experienced persistent heavy snowfall with unusu- ally low temperatures. ESEs can be divided into 3 groups according to the geographical coverage of snowfall, using the following criteria to define an ESE: >15 snowfall days, 20 snow-cover/icing days, and 30 cm total cumulated snow depth for an individual winter. The first group covers the whole of Eastern China (East of 105° E), and ESEs occurred in 1654, 1660, 1665, 1670, 1676, 1683, 1689, 1690, 1700, 1714, 1719, 1830−32, 1840, 1877 and 1892; the second group is located mainly in the area south of Huaihe River (~33° N), and ESEs occured in 1694, 1887, 1929, and 1930; and the third group is confined within the central region between Yellow River and Nanling Mountain (roughly 26° to 35° N), and ESEs occurred in 1578, 1620, 1796, and 1841. -

Huaxin Cement Co., Ltd. Annual Report 2017
Huaxin Cement Co., Ltd. 600801 Annual Report 2017 1 Important Notice I. The Board of Directors of the Company and its members, the Board of Supervisors of the Company and its members and Top Management members confirm, to the best of their knowledge, that there is no false or misleading statement or material omission in this report and shall be severally and jointly liable for the truthfulness, accuracy and completeness of its contents. II. All the Directors attended the Board Meeting. III. PricewaterhouseCoopers Zhong Tian CPAs LLP issued standard audit report with unmodified opinion for the Company. IV. Chairman of the Company Mr. Xu Yongmo, Legal Representative and CEO Mr. Li Yeqing, person in charge of accounting Ms. Kong Lingling, and Chief of Accounting Department Mr. Wu Xin declare and confirm that the Financial Statements contained in the Annual Report are true, accurate and complete. V. Profit distribution proposal for the reporting period reviewed by the Board of Directors In 2017, the Parent Company achieved net profit of 1,728,197,485 Yuan or 2,077,640,568 Yuan net profit attributable to the shareholders after consolidation. Pursuant to the relevant provisions contained in the Company Law and the Accounting Rule, 10%, i.e. 172,819,749 Yuan will be appropriated to statutory surplus common reserve fund. The allocable profit of the Parent Company is 4,415,356,360 Yuan by the end of December 2017. The Board proposes that on the basis of the total 1,497,571,325 shares, a cash dividend of 0.28 Yuan per share (incl. -

Spermatophyte Flora of Liangzi Lake Wetland Nature Reserve
E3S Web of Conferences 143, 02040 (2 020) https://doi.org/10.1051/e3sconf/20 2014302040 ARFEE 2019 Spermatophyte Flora of Liangzi Lake Wetland Nature Reserve Xinyang Zhang, Shijing He*, Rong Tao, and Huan Dai Wuhan Institute of Design and Sciences, Wuhan 430205, China Abstract. Based on route and sample-plot survey, plant resources of Liangzi Lake Wetland Nature Reserve were investigated. The result showed that there were 503 species of spermatophyte belonging to 296 genera of 86 families. There were 5 species under national first and second level protection. The dominant families of spermatophyte contained 20 species and above. The dominant genera of spermatophyte contained 4 species and below. The 86 families of spermatophyte can be divided into 7 distribution types and 4 variants. Tropic distribution type was dominant, accounting for 70.83% in total (excluding cosmopolitans). The 296 genera of spermatophyte can be divided into 14 distribution types and 9 variants. Temperate elements were a little more than tropical elements, accounting for 50.84% and 49.16% in total (excluding cosmopolitans) respectively. Reserve had 3 Chinese endemic genera, reflecting certain ancient and relict. The purpose of the research is to provide background information and scientific basis for the protection, construction, management and rational utilization of plant resources in the reserve. 1 Preface temperature is 17℃, the annual average rainfall is 1663mm, the average sunshine hour is 2061 hours, and Flora refers to the sum of all plant species in a certain the frost free period is 270 days. The rain bearing area is region or country. It is the result of the development and 208500 hectares, and the annual average water level is evolution of the plant kingdom under certain natural and 17.81m. -

Epidemiological Survery on Clonorchiasis Sinensis in Yangxin
EPIDEMIOLOGICAL SURVEY ON CLONORCHIASIS SINENSIS IN YANGXIN COUNTY OF HUBEI PROVINCE OF PR CHINA 3 Sili Chen', Siyi Cherr', Fengjiao Wu2, Sizhi Cherr', Shanquan Ke , Shunzhi Cherr' and Zichun Pan' 1 Hubei Provincial Academy of Medical Sciences, Wuchang, Wuhan, Hubei Province, 430070, People's Republic of China; 2Daqiao Hospital, Yangxin County of Hubei Province, Yangxin 435242, PR China; 3Health And Anti-epidemic Station of Daye City of Hubei Province, Daye 435100, PR China Abstract. An epidemiological survey of clonorchiasis was conducted at Panqiao township of Yangxin County of Hubei Province from June to November, 1993. The positive rate of cercaria in the body of intermediate hosts, Parafossarulus stratulus and Alocinma longicornis was 12.25% and 3.84% respectively. Positive rates of metacercariae in the bodies of Pseudonaphona parva was 48.15%, Ctenopharyngodon idellus 17.24% and Hypophthalmichthys nobilis 18.18%. Positive rate of eggs in the feces of cats was 36.36% and pigs 16.67%. It has been confirmed that there is a natural focus of clonorchiasis sinensis at Yangxin County of Hubei Province. A total population of 6,865 in 20 sites of 10 production brigades of Panqiao township was surveyed for infection with Clonorchis sinensis. The average infection rate in the local residents was 5.80%. Male had a higher infection rate than female. The infected persons were mainly peasants and school girls and boys. Most of the infected persons had light infections ( 1°)without a serious clinical manifestations. INTRODUCTION Study target 1. All residents over 5 years old were the targets Eggs of Clonorchis sinensis were found in feces of this study. -

Variety Identification and Landscape Application of Osmanthus Fragrans
OPEN ACCESS Freely available online l of Hortic na ul r tu u r o e J Journal of Horticulture ISSN: 2376-0354 Rapid Communication Variety Identification and Landscape Application of Osmanthus fragrans in Jingzhou City Hongna Mu, Fei Zhao, Dongling Li, Taoze Sun* College of Horticulture and Gardening, Yangtze University, No. 88 Jingmi Road, Jingzhou, Hubei 434025, China ABSTRACT Osmanthus fragrans had been considered one of the most popular trees in China for its economic value and cultural significance. O. fragrans, the most ubiquitous garden plants, which varieties had been unclear in Jingzhou city. It is an urgent task to figure out the precise varieties. Therefore, the investigation was carried out. Finally, 24 varieties were identifiedand landscape application had been illustrated in this paper. Keywords: Osmanthus fragrans; Variety identification; Landscape application INTRODUCTION Jingzhou city, the capital of Chu Dynasty (existed 740-223 B.C.), located at the east longitude 111ºC and 14ºC the North latitude Osmanthus fragrans, commonly known as sweet osmanthus, is a 29ºC and 37ºC, an important highway transportation hub and a woody, evergreen species of shrubs and small trees in the Oleaceae port city along the Yangtze River, the total area is 141,000 square family. Sweet osmanthus has considerable economic value and kilometers, in which 78.7% are plain lakes, is affiliated to Hubei cultural significance and is one of the top ten traditional flowers Province. Jingzhou is a subtropical monsoon climate area. Both in China. Twenty-four of the thirty-five species in the Osmanthus sunlight energy and heat are abundant, frost-free period up to genus are distributed in China, with the mostly highly representative 242-263 days. -

Comparing Chinese and United States Lake Management and Protection As Shared Through a Sister Lakes Program
Comparing Chinese and United States lake management and protection As shared through a sister lakes program Lake Pepin, Minnesota Liangzi Lake, Hubei Province Minnesota Pollution May 2016 Control Agency Authors Steven Heiskary Research Scientist III Water Quality Monitoring Unit Minnesota Pollution Control Agency, Zhiquan Chen Manager Hubei Province Department of Environmental Protection Yiluan Dong Environmental Protection Engineer Hubei Province Department of Environmental Protection Review Pam Anderson Water Quality Monitoring Unit Minnesota Pollution Control Agency Authors Steven Heiskary, Research Scientist III, Water Quality Monitoring Unit, Minnesota Pollution Control Agency Zhiquan Chen, Manager, Hubei Province Department of Environmental Protection Yiluan Dong, Registered Environmental Protection Engineer, Hubei Academy of Environmental Sciences, China Review Pam Anderson, Water Quality Monitoring Unit, Minnesota Pollution Control Agency Editing and graphic design Theresa Gaffey & Paul Andre Minnesota Pollution Control Agency 520 Lafayette Road North | Saint Paul, MN 55155-4194 | | 800-657-3864 | Or use your preferred relay service. [email protected] 651-296-6300 | This report is available in alternative formats upon request, and online at www.pca.state.mn.us. Document number: wq-s1-91 Contents Abstract ........................................................................................................................................................ 1 Introduction ........................................................................................................................................................ -

From Bloomery Furnace to Blast Furnace Archeometallurgical Analysis of Medieval Iron Objects from Sigtuna and Lapphyttan, Sverige
EXAMENSARBETE INOM TEKNIK, GRUNDNIVÅ, 15 HP STOCKHOLM, SVERIGE 2019 From Bloomery Furnace to Blast Furnace Archeometallurgical Analysis of Medieval Iron Objects From Sigtuna and Lapphyttan, Sverige ANDREAS HELÉN ANDREAS PETTERSSON KTH SKOLAN FÖR INDUSTRIELL TEKNIK OCH MANAGEMENT Abstract During the Early Middle Ages, the iron production in Sweden depended on the bloomery furnace, which up to that point was well established as the only way to produce iron. Around the Late Middle Ages, the blast furnace was introduced in Sweden. This made it possible to melt the iron, allowing it to obtain a higher carbon composition and thereby form new iron-carbon phases. This study examines the microstructure and hardness of several tools and objects originating from archaeological excavations of Medieval Sigtuna and Lapphyttan. The aim is to examine the differences in quality and material properties of iron produced by respectively blast furnaces and bloomery furnaces. Both methods required post-processing of the produced iron, i.e. decarburization for blast furnaces and carburization for bloomeries. These processes were also studied, to better understand why and how the material properties and qualities of the items may differ. The results show that some of the studied items must have been produced from blast furnace iron, due to their material composition and structure. These items showed overall better material quality and contained less slag. This was concluded because of the increased carbon concentration that allowed harder and more durable structures such as pearlite to form. The study also involved an investigation of medieval scissors, also known as shears, made from carburized bloomery furnace iron.