Bloomery (Edited from Wikipedia)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Register of Historic Places Multiple Property
NFS Form 10-900-b 0MB No. 1024-0018 (Jan. 1987) United States Department of the Interior National Park Service National Register of Historic Places Multipler Propertyr ' Documentation Form NATIONAL This form is for use in documenting multiple property groups relating to one or several historic contexts. See instructions in Guidelines for Completing National Register Forms (National Register Bulletin 16). Complete each item by marking "x" in the appropriate box or by entering the requested information. For additional space use continuation sheets (Form 10-900-a). Type all entries. A. Name of Multiple Property Listing ____Iron and Steel Resources of Pennsylvania, 1716-1945_______________ B. Associated Historic Contexts_____________________________ ~ ___Pennsylvania Iron and Steel Industry. 1716-1945_________________ C. Geographical Data Commonwealth of Pennsylvania continuation sheet D. Certification As the designated authority under the National Historic Preservation Act of 1966, as amended, J hereby certify that this documentation form meets the National Register documentation standards and sets forth requirements for the listing of related properties consistent with the National Register criteria. This submission meets the procedural and professional requiremerytS\set forth iri36JCFR PafrfsBOfcyid the Secretary of the Interior's Standards for Planning and Evaluation. Signature of certifying official Date / Brent D. Glass Pennsylvania Historical & Museum Commission State or Federal agency and bureau I, hereby, certify that this multiple -
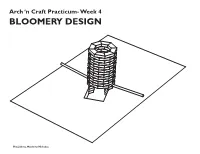
Bloomery Design
Arch ‘n Craft Practicum- Week 4 BLOOMERY DESIGN Hiu, Julieta, Matthew, Nicholas 7 3/4" The Stack: 3 3/4" In our ideal model we would use refractory bricks, either rectangular bricks or curved bricks which would help us make a circular 1'-8" stack. The stack would be around 3 feet tall with a 1 foot diameter for the inner wall of the stack. There will be openings about 3 brick lengths up(6-9 inches) from the ground on 1'-0" opposite ends of the stack. Attached to these openings will be our tuyeres which in turn will connect to our air sources. Building a circular stack would be beneficial to the type of air distribution we want to create within our furnace; using two angled tuyeres we would like air to move clockwise and counterclock- Bloomery Plan wise. We would like to line the inside of the stack with clay to provide further insulation. Refractory bricks would be an ideal material to use because the minerals they are com- posed of do not change chemically when 1'-0" exposed to high heat, some examples of these materials are aluminum, manganese, silica and zirconium. Other comments: 3'-0" Although we have opted to use refractory bricks, which would be practical for our intended purpose, we briefly considered using Vycor glass which can withstand high tem- peratures of up to 900 degrees celsius or approximately 1652 degrees Fahrenheit, which Tuyere height: Pitched -10from horizontal 7" would be enough for our purposes. Bloomery Section Ore/ Charcoal: Our group has decided to use Magnetite ore along with charcoal for the smelt. -

Pig Iron Sub-Committee, Chaired by Rodrigo Valladares, CEO of Viena Siderúrgica S/A, for Preparing and Editing the Information Presented in This Guide
Copyright © 2014 by International Iron Metallics Association Ltd. This guide published by International Iron Metallics Association Ltd. All rights reserved. This guide may be used or reproduced in any manner whatsoever providing it includes full acknowledgement to the IIMA. 2011 International Iron Metallics Association (IIMA) Printed and bound in the United Kingdom Disclaimer This guide is intended for information purposes only and is not intended as commercial material in any respect. The material is not intended as an offer or solicitation for the purposes of sale of any financial instrument, is not intended to provide an investment recommendation and should not be relied upon for such. The material is derived from published sources, together with personal research. No responsibility or liability is accepted for any such information of opinions or for any errors, omissions, misstatements, negligence, or otherwise for any further communication, written or otherwise. ii ACKNOWLEDGEMENT The International Iron Metallics Association (IIMA) wishes to thank Dr. Oscar Dam, Chief Technical Advisor, and the Pig Iron Sub-Committee, chaired by Rodrigo Valladares, CEO of Viena Siderúrgica S/A, for preparing and editing the information presented in this guide. FOREWORD The International Iron Metallics Association was created to promote the use of ore-based metallics (pig iron, HBI, DRI, and iron nuggets) as value-adding raw materials for the iron and steel and ferrous casting industries. Safe and efficient handling and shipping of merchant ore-based metallics are vital to the commercial trade and use of these materials. Therefore, we are continuing the series of guides begun by IIMA co-founder, HBI Association (HBIA), with this guide which addresses the methods, techniques, and procedures for handling and transferring merchant pig iron at dry bulk terminals. -

Primary Mill Fabrication
Metals Fabrication—Understanding the Basics Copyright © 2013 ASM International® F.C. Campbell, editor All rights reserved www.asminternational.org CHAPTER 1 Primary Mill Fabrication A GENERAL DIAGRAM for the production of steel from raw materials to finished mill products is shown in Fig. 1. Steel production starts with the reduction of ore in a blast furnace into pig iron. Because pig iron is rather impure and contains carbon in the range of 3 to 4.5 wt%, it must be further refined in either a basic oxygen or an electric arc furnace to produce steel that usually has a carbon content of less than 1 wt%. After the pig iron has been reduced to steel, it is cast into ingots or continuously cast into slabs. Cast steels are then hot worked to improve homogeneity, refine the as-cast microstructure, and fabricate desired product shapes. After initial hot rolling operations, semifinished products are worked by hot rolling, cold rolling, forging, extruding, or drawing. Some steels are used in the hot rolled condition, while others are heat treated to obtain specific properties. However, the great majority of plain carbon steel prod- ucts are low-carbon (<0.30 wt% C) steels that are used in the annealed condition. Medium-carbon (0.30 to 0.60 wt% C) and high-carbon (0.60 to 1.00 wt% C) steels are often quenched and tempered to provide higher strengths and hardness. Ironmaking The first step in making steel from iron ore is to make iron by chemically reducing the ore (iron oxide) with carbon, in the form of coke, according to the general equation: Fe2O3 + 3CO Æ 2Fe + 3CO2 (Eq 1) The ironmaking reaction takes place in a blast furnace, shown schemati- cally in Fig. -

The Future of Steelmaking– Howeuropean the MANAGEMENT SUMMARY
05.2020 The future of steelmaking – How the European steel industry can achieve carbon neutrality MANAGEMENT SUMMARY The future of steelmaking / How the European steel industry can achieve carbon neutrality The European steelmaking industry emits 4% of the EU's total CO2 emissions. It is under growing public, economic and regulatory pressure to become carbon neutral by 2050, in line with EU targets. About 60% of European steel is produced via the so-called primary route, an efficient but highly carbon-intensive production method. The industry already uses carbon mitigation techniques, but these are insufficient to significantly reduce or eliminate carbon emissions. The development and implementation of new technologies is underway. With limited investment cycles left until the 2050 deadline, the European steelmaking industry must decide on which new technology to invest in within the next 5-10 years. We assess the most promising emerging technologies in this report. They fall into two main categories: carbon capture, use and/or storage (CCUS), and alternative reduction of iron ore. CCUS processes can be readily integrated into existing steel plants, but cannot alone achieve carbon neutrality. If biomass is used in place of fossil fuels in the steelmaking process, CCUS can result in a negative carbon balance. Alternative reduction technologies include hydrogen-based direct reduction processes and electrolytic reduction methods. Most are not well developed and require huge amounts of green energy, but they hold the promise of carbon-neutral steelmaking. One alternative reduction process, H2-based shaft furnace direct reduction, offers particular promise due to its emissions-reduction potential and state of readiness. -

Comparative Properties of Wrought Iron Made by Hand Puddling and by the Aston Process
RP124 COMPARATIVE PROPERTIES OF WROUGHT IRON MADE BY HAND PUDDLING AND BY THE ASTON PROCESS By Henry S. Rawdon and 0. A. Knight ABSTRACT The hand-puddling method of making wrought iron has not greatly changed for a century. More economical methods in the manufacture of thjs product is the crying need of the industry. A radically new process, recently developed, is now coming into commercial use, in which pig iron, which h>as been refined in a Bessemer converter, is poured into molten slag so as to produce intimate mingling of the two. A comparison of the properties of wrought iron made thus with that made by hand puddling forms the subject of this report. The test results failed to show any marked difference in the products of the two processes. The new product appears to have all of the essential properties usually connoted by the name—wrought iron, CONTENTS Page I. Introduction 954 1. Resume of the Aston process 955 II. Purpose and scope of the investigation 959 III. Materials and methods 960 1. Materials 960 (a) Pipe 960 (6) "Rounds" 961 (c) Slag 962 2. Methods 962 IV. Results 962 1. Composition 962 2. Density 964 3. Mechanical properties 965 (a) Pipe materials 965 (1) Tensile properties 965 (2) Torsional properties 970 (3) Flattening tests 971 (6) 1-inch rounds 972 (1) Tensile properties 972 (2) Torsional properties 973 (3) Impact resistance 973 4. Corrosion resistance 976 (a) Laboratory corrosion tests 976 (6) Electrolytic solution potential 979 5. Structural examination 979 (a) Pipe materials 980 (1) BaU 980 (2) Muck bar 980 (3) Skelp 981 (4) Pipe 981 (&) 1-inch rounds 981 (c) Slag 981 953 : 954 Bureau of Standards Journal of Research [vol. -

Final Exam Questions Generated by the Class
Final Exam Questions Generated by the Class Module 8 – Iron and Steel Describe some of the business practices that Carnegie employed that allowed him to take command of the steel industry. Hard driving, vertical integration, price making Which of the following was/is NOT a method used to make steel? A. Puddling B. Bessemer process C. Basic oxygen process D. Arc melting E. None of the above What are the three forms of iron, and what is the associated carbon content of each? Wrought <.2% Steel .2-2.3% Cast Iron 2.3-4.2% How did Andrew Carnegie use vertical integration to gain control of the steel market? Controlled the entire steel making process from mining to final product Who created the best steel for several hundred years while making swords during the 1500’s? A. Syria B. Egypt C. Japan D. England Describe the difference between forging and casting. When forging, you beat and hammer the material into the desired shape. When casting, you pour liquid into a mold to shape it. Describe the difference between steel and wrought iron. Steel has less carbon Which of the following forms of iron has a low melting point and is not forgeable? A. Steel B. Pig Iron C. Wrought Iron D. None of the Above What two developments ushered in the transition from the Bronze Age to the Iron Age? More iron ore and greater ability to change its properties using readily available alloying agent (carbon) 1 Final Exam Questions Generated by the Class What is the difference between ferrite and austenite? A. -

Historical Survey of Iron and Steel Production in Bosnia and Herzegovina
UDK 669.1(497.15)(091) ISSN 1580-2949 Professional article/Strokovni ~lanek MTAEC9, 43(4)223(2009) S. MUHAMEDAGI], M. ORU]: HISTORICAL SURVEY OF IRON AND STEEL PRODUCTION IN BiH HISTORICAL SURVEY OF IRON AND STEEL PRODUCTION IN BOSNIA AND HERZEGOVINA ZGODOVINSKI PREGLED PROIZVODNJE @ELEZA IN JEKLA V BOSNI IN HERCEGOVINI Sulejman Muhamedagi}1, Mirsada Oru~2 1University of Zenica, Faculty of metallurgy and materials, Travni~ka c. 1, 72000 Zenica, Bosna i Hercegovina 2University of Zenica, Institute of Metallurgy "Kemal Kapetanovi}", Travni~ka c. 1, 72000 Zenica, Bosna i Hercegovina [email protected] Prejem rokopisa – received: 2009-01-08; sprejem za objavo – accepted for publication: 2009-01-16 Cast-iron and steel production facilities were, and still are, frequently located on sites with deposits of iron ore and coal. The center of steel metallurgy in Bosnia and Herzegovina, and of the former Yugoslavia, is located in the Iron and Steel Plant Zenica, today known as Arcelor Mittal Zenica. In this paper the beginning, the development and the planned growth of the iron and steel plant in Zenica is presented with periods of success and periods of crisis. Key words: Iron and Steel Plant Zenica, developmentr, pig iron, steel. Proizvodne naprave za grodelj in jeklo so pogosto zgrajene na le`i{~ih `elezove rude in premoga. Sredi{~e proizvodnje jekla v Bosni in Hercegovini ter v nekdanji Jugoslaviji je bilo v @elezarni Zenica, danes Arcelor Mittal Zenica. V tem sestavku so predstavljeni za~etek, razvoj in na~rtovana rast @elezarne Zenica z obdobji krize in uspeha. Klju~ne besede: @elezarna Zenica, razvoj, grodelj, jeklo advantage of road and railway communications along the 1 INTRODUCTION Bosna valley. -

Download Newsletter
THE HOT IRON SPARKLE * Newsletter of the North Carolina ABANA * www.ncabana.org Volume 29 Number 1 1st Quarter 2011 – Jan/Feb/Mar Peter Ross Working With Wrought Iron Inside This Issue Interview With Peter Ross – President’s Message P 2 P 17 Fire On the Mountain P 31 Wrought Iron The Last Blacksmith Standing Editor’s Notes P 3 P 24 2011 SBA Conference P 32 – Chuck Beattie Blacksmith’s Guild of the Controlled Hand Forging – Secretary’s Report P 3 P 25 P 34 Potomac Spring Fling Splitting Meet our New Treasurer – The Stirling Cycle Engine – New England School of P 4 P 26 P 38 Jim Kennady Robert Timberlake Metalwork Summer Classes Treasurer’s Report P 4 Wanted: Blacksmith Staff P 27 Blacksmith’s Exchange P 39 Book Review – The Backyard Regional Group Meetings P 5 P 28 Forms P 42 Blacksmith Letter To The Editor P 12 Bill Tannenberg’s Latest P 29 2011 Chapter Calendar P 43 Forth Quarter Chapter Yadkinville Journeyman – P 13 P 30 New Members P 44 Meeting Allan Green Upcoming Chapter Meetings P 16 Chapter Officers P 44 Page 2 of 44 * THE HOT IRON SPARKLE * Volume 29, No. 1 A Message from Our President PRESIDENT’S LETTER The chapter held our 4th quarter meeting at Peter Ross’s shop with Peter demonstrating to an attentive crowd. Lunch was cooked by Jim Kennady with no one leaving hungry. Thanks to the Ross’s for hosting us. We held our elections at the last quarter meeting with Jim Kennady being elected as treasurer and myself as your president. -

From Bloomery Furnace to Blast Furnace Archeometallurgical Analysis of Medieval Iron Objects from Sigtuna and Lapphyttan, Sverige
EXAMENSARBETE INOM TEKNIK, GRUNDNIVÅ, 15 HP STOCKHOLM, SVERIGE 2019 From Bloomery Furnace to Blast Furnace Archeometallurgical Analysis of Medieval Iron Objects From Sigtuna and Lapphyttan, Sverige ANDREAS HELÉN ANDREAS PETTERSSON KTH SKOLAN FÖR INDUSTRIELL TEKNIK OCH MANAGEMENT Abstract During the Early Middle Ages, the iron production in Sweden depended on the bloomery furnace, which up to that point was well established as the only way to produce iron. Around the Late Middle Ages, the blast furnace was introduced in Sweden. This made it possible to melt the iron, allowing it to obtain a higher carbon composition and thereby form new iron-carbon phases. This study examines the microstructure and hardness of several tools and objects originating from archaeological excavations of Medieval Sigtuna and Lapphyttan. The aim is to examine the differences in quality and material properties of iron produced by respectively blast furnaces and bloomery furnaces. Both methods required post-processing of the produced iron, i.e. decarburization for blast furnaces and carburization for bloomeries. These processes were also studied, to better understand why and how the material properties and qualities of the items may differ. The results show that some of the studied items must have been produced from blast furnace iron, due to their material composition and structure. These items showed overall better material quality and contained less slag. This was concluded because of the increased carbon concentration that allowed harder and more durable structures such as pearlite to form. The study also involved an investigation of medieval scissors, also known as shears, made from carburized bloomery furnace iron. -

GREEN STEEL INSIGHT BRIEF September 2019
M OUN KY T C A I O N THE DISRUPTIVE POTENTIAL OF R I N E STIT U T GREEN STEEL INSIGHT BRIEF September 2019 IIIIIIII INSIGHT SUMMARY Thomas Koch Blank • Because the steel industry is one of the largest emitters of CO2, contributing 6%–7% of Rocky Mountain Institute global greenhouse gas emissions, it is imperative to find a low-carbon process for primary [email protected] steelmaking in order to adhere to a 1.5°C pathway. • Several non-coal-based technologies for primary steelmaking are currently being piloted, with hydrogen-based direct reduction being the furthest developed. • Using today’s global average grid power, primary steel production with hydrogen has the same CO2 footprint as the highest-performing installations of the prevailing blast furnace method. • In the United States and European Union, switching to hydrogen-based steel production represents an immediate CO2 emissions reduction opportunity for new assets of 20% and 40% respectively. • Given that prices of electricity and coking coal are not coupled, the 20% cost premium of hydrogen-based steel production is eliminated at electricity prices of $15–$20/MWh or lower, a cost level achieved already today by renewable power plants across several geographies (e.g., Brazil, Mexico, Saudi Arabia, Portugal and the United States). • While this sounds promising, adding up all of the steel mills from all of the companies currently investing in or committing to zero-carbon technology only represent 8% of global steel production. A 100-fold step-change in the pace of transition is required to adhere to a 1.5°C pathway, which will require a concert of market, regulatory, and finance interventions to accelerate deployment of low-carbon technologies. -

The Production of High Carbon Steel Directly in Bloomery Process: Theoretical Bases and Metallographic Analyses of the Experiments Results
EXARC JOURNAL, ISSUE 2013/2 The Production of High Carbon Steel Directly in Bloomery Process: Theoretical Bases and Metallographic Analyses of the Experiments Results Adrian Wrona (PL) The problem of steel1 making in antiquity has intrigued researchers who specialize in ancient metallurgy for decades. In the course of research different explanations have prevailed in the scholarship. R. J. Forbes (1950, 405-412) in his classic work Metallurgy in Antiquity distinguished three main methods of steel production: 1. Steel obtained directly in the bloomery process. However, he points out that high carbon steel was only a small part of smelted bloom and was not able to fulfil the population's needs for a high-quality material used to produce the necessary tools. He argued his thesis by poor ores used by ancient smelters, low temperature of process, et cetera. 2. As a second method, Forbes mentions the possibility of decarburization of cast iron obtained during the smelting process, but he rejects the intentional receive of it in the reduction process. The fragments of pig iron occasionally found, mostly on the Roman metallurgical sites, are (according to Forbes) accidental by-products that have been abandoned as an useless and not suitable for further processing. 3. A third possibility is making steel by carburization of soft iron, commonly called cementation or casehardening. This distinction came to form the basis our knowledge on the subject, and these methods became a basis for further studies and explanations about carburizing technique used in tool and weapon production. For a long time the third possibility was considered the main method for the preparation .