Mechanical Properties of Medieval Bloomery Iron Materials 2015 59 1 35 Table 1 Samples for Mechanical Testing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Carburization Time and Temperature on the Mechanical Properties of Carburized Mild Steel, Using Activated Carbon As Carburizer
Materials Research, Vol. 12, No. 4, 483-487, 2009 © 2009 Effects of Carburization Time and Temperature on the Mechanical Properties of Carburized Mild Steel, Using Activated Carbon as Carburizer Fatai Olufemi Aramidea,*, Simeon Ademola Ibitoyeb, Isiaka Oluwole Oladelea, Joseph Olatunde Borodea aMetallurgical and Materials Engineering Department, Federal University of Technology, Akure, Ondo State, Nigeria bMaterials Science and Engineering Department, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria Received: July 31, 2009; Revised: September 25, 2009 Due to the complexity of controlling parameters in carburization, there has been relatively little work on process variables during the surface hardening process. This work focuses on the effects of the carburizing temperature and time on the mechanical properties of mild steel carburized with activated carbon, at 850, 900 and 950 °C, soaked at the carburizing temperature for 15 and 30 minutes, quenched in oil, tempered at 550 °C and held for 60 minutes. Prior carburization process, standard test samples were prepared from the as received specimen for tensile and impact tests. After carburization process, the test samples were subjected to the standard test and from the data obtained, ultimate tensile strength, engineering strain, impact strength, Youngs’ moduli were calculated. The case and core hardness of the carburized tempered samples were measured. It was observed that the mechanical properties of mild steels were found to be strongly influenced by the process of carburization, carburizing temperature and soaking time at carburizing temperature. It was concluded that the optimum combination of mechanical properties is achieved at the carburizing temperature of 900 °C followed by oil quenching and tempering at 550 °C. -

National Register of Historic Places Multiple Property
NFS Form 10-900-b 0MB No. 1024-0018 (Jan. 1987) United States Department of the Interior National Park Service National Register of Historic Places Multipler Propertyr ' Documentation Form NATIONAL This form is for use in documenting multiple property groups relating to one or several historic contexts. See instructions in Guidelines for Completing National Register Forms (National Register Bulletin 16). Complete each item by marking "x" in the appropriate box or by entering the requested information. For additional space use continuation sheets (Form 10-900-a). Type all entries. A. Name of Multiple Property Listing ____Iron and Steel Resources of Pennsylvania, 1716-1945_______________ B. Associated Historic Contexts_____________________________ ~ ___Pennsylvania Iron and Steel Industry. 1716-1945_________________ C. Geographical Data Commonwealth of Pennsylvania continuation sheet D. Certification As the designated authority under the National Historic Preservation Act of 1966, as amended, J hereby certify that this documentation form meets the National Register documentation standards and sets forth requirements for the listing of related properties consistent with the National Register criteria. This submission meets the procedural and professional requiremerytS\set forth iri36JCFR PafrfsBOfcyid the Secretary of the Interior's Standards for Planning and Evaluation. Signature of certifying official Date / Brent D. Glass Pennsylvania Historical & Museum Commission State or Federal agency and bureau I, hereby, certify that this multiple -
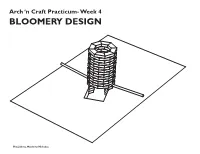
Bloomery Design
Arch ‘n Craft Practicum- Week 4 BLOOMERY DESIGN Hiu, Julieta, Matthew, Nicholas 7 3/4" The Stack: 3 3/4" In our ideal model we would use refractory bricks, either rectangular bricks or curved bricks which would help us make a circular 1'-8" stack. The stack would be around 3 feet tall with a 1 foot diameter for the inner wall of the stack. There will be openings about 3 brick lengths up(6-9 inches) from the ground on 1'-0" opposite ends of the stack. Attached to these openings will be our tuyeres which in turn will connect to our air sources. Building a circular stack would be beneficial to the type of air distribution we want to create within our furnace; using two angled tuyeres we would like air to move clockwise and counterclock- Bloomery Plan wise. We would like to line the inside of the stack with clay to provide further insulation. Refractory bricks would be an ideal material to use because the minerals they are com- posed of do not change chemically when 1'-0" exposed to high heat, some examples of these materials are aluminum, manganese, silica and zirconium. Other comments: 3'-0" Although we have opted to use refractory bricks, which would be practical for our intended purpose, we briefly considered using Vycor glass which can withstand high tem- peratures of up to 900 degrees celsius or approximately 1652 degrees Fahrenheit, which Tuyere height: Pitched -10from horizontal 7" would be enough for our purposes. Bloomery Section Ore/ Charcoal: Our group has decided to use Magnetite ore along with charcoal for the smelt. -

History of the Hardening of Steel : Science and Technology J
HISTORY OF THE HARDENING OF STEEL : SCIENCE AND TECHNOLOGY J. Vanpaemel To cite this version: J. Vanpaemel. HISTORY OF THE HARDENING OF STEEL : SCIENCE AND TECHNOLOGY. Journal de Physique Colloques, 1982, 43 (C4), pp.C4-847-C4-854. 10.1051/jphyscol:19824139. jpa- 00222126 HAL Id: jpa-00222126 https://hal.archives-ouvertes.fr/jpa-00222126 Submitted on 1 Jan 1982 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. JOURNAL DE PHYSIQUE Colloque C4, suppZ4ment au no 12, Tome 43, de'cembre 1982 page C4-847 HISTORY OF THE HARDENING OF STEEL : SCIENCE AND TECHNOLOGY 3. Vanpaemel Center for historical and socio-economical studies on science and technology Teer EZstZaan 41, 3030 Leuven, SeZgim (Accepted 3 November 1982) Abstract. - The knowledge of the hardening phenomenon was achieved through a very cumulative process without any dis- continuity or 'scientific crisis' . The history of the hardening shows a definite interrelationship between techno- logical approach (or the application-side) and academic science . The hardening of steel appears to have been an operation in common use among the early Greeks The Greek and Roman smiths knew, from experience, how to control the. -

Guide to Non-Ferrous Metals
14 Manufacturing Processes CHAPTER 2 Ferrous Materials and Non-Ferrous Metals and Alloys 2.1 INTRODUCTION Ferrous materials/metals may be defined as those metals whose main constituent is iron such as pig iron, wrought iron, cast iron, steel and their alloys. The principal raw materials for ferrous metals is pig iron. Ferrous materials are usually stronger and harder and are used in daily life products. Ferrous material possess a special property that their characteristics can be altered by heat treatment processes or by addition of small quantity of alloying elements. Ferrous metals possess different physical properties according to their carbon content. 2.2 IRON AND STEEL The ferrous metals are iron base metals which include all varieties of iron and steel. Most common engineering materials are ferrous materials which are alloys of iron. Ferrous means iron. Iron is the name given to pure ferrite Fe, as well as to fused mixtures of this ferrite with large amount of carbon (may be 1.8%), these mixtures are known as pig iron and cast iron. Primarily pig iron is produced from the iron ore in the blast furnace from which cast iron, wrought iron and steel can be produced. 2.3 CLASSIFICATION OF CARBON STEELS Plain carbon steel is that steel in which alloying element is carbon. Practically besides iron and carbon four other alloying elements are always present but their content is very small that they do not affect physical properties. These are sulphur, phosphorus, silicon and manganese. Although the effect of sulphur and phosphorus on properties of steel is detrimental, but their percentage is very small. -

Ore, Iron, Artefacts and Corrosion
. SERIE C NR 626 AVHANDLINGAR OCH UPPSATSER ARSBOK 61 NR 11 OLOF ARRHENIUS ORE, IRON, ARTEFACTS AND CORROSION WITH 4 PLATES STOCKHOLM 1967 SVERTGES GEOLOGISKA UNDERSOKNING SERIE C NR 626 ARSBOK 6 l NR I I OLOF ARRHENIUS ORE, IRON, ARTEFACTS AND CORROSION WITH 4 PLATES STOCKHOLM 196 7 Contents The conditions of the investigation ....................... Elements on which studies are made. ...................... Methods and sources of material ........................ Conditions of analysis ............................ What ores were first used in the production of iron? ................ The formation, occurrence and chemical composition of limonite ores ........ Synopsis of regions in which lake iron ore has a relatively high frequency of high con- centration of the elements indicated ...................... Rock ores. ................................. Synopsis of regions in which rock ores have a relatively high frequency of high concentra- tions of the elements indicated ........................ Artefacts .................................. Synopsis of regions where artefacts show relatively high frequencitl of the elements indicated ................................. Phosphorus in iron ............................. Coal in iron ............................... Alloying elements. Rust ........................... Summary .................................. Appendix: The microstructure after reduction of phosphorus-rich iron ore with charcoal at different temperatures. By Torsten Hansson and Sten Modin .......... Literature. ................................ -

Comparative Properties of Wrought Iron Made by Hand Puddling and by the Aston Process
RP124 COMPARATIVE PROPERTIES OF WROUGHT IRON MADE BY HAND PUDDLING AND BY THE ASTON PROCESS By Henry S. Rawdon and 0. A. Knight ABSTRACT The hand-puddling method of making wrought iron has not greatly changed for a century. More economical methods in the manufacture of thjs product is the crying need of the industry. A radically new process, recently developed, is now coming into commercial use, in which pig iron, which h>as been refined in a Bessemer converter, is poured into molten slag so as to produce intimate mingling of the two. A comparison of the properties of wrought iron made thus with that made by hand puddling forms the subject of this report. The test results failed to show any marked difference in the products of the two processes. The new product appears to have all of the essential properties usually connoted by the name—wrought iron, CONTENTS Page I. Introduction 954 1. Resume of the Aston process 955 II. Purpose and scope of the investigation 959 III. Materials and methods 960 1. Materials 960 (a) Pipe 960 (6) "Rounds" 961 (c) Slag 962 2. Methods 962 IV. Results 962 1. Composition 962 2. Density 964 3. Mechanical properties 965 (a) Pipe materials 965 (1) Tensile properties 965 (2) Torsional properties 970 (3) Flattening tests 971 (6) 1-inch rounds 972 (1) Tensile properties 972 (2) Torsional properties 973 (3) Impact resistance 973 4. Corrosion resistance 976 (a) Laboratory corrosion tests 976 (6) Electrolytic solution potential 979 5. Structural examination 979 (a) Pipe materials 980 (1) BaU 980 (2) Muck bar 980 (3) Skelp 981 (4) Pipe 981 (&) 1-inch rounds 981 (c) Slag 981 953 : 954 Bureau of Standards Journal of Research [vol. -

Historical Iron by CLARA DECK, CONSERVATOR REVISIONS by LOUISE BECK, CONSERVATOR
The Care and Preservation Of Historical Iron BY CLARA DECK, CONSERVATOR REVISIONS BY LOUISE BECK, CONSERVATOR Introduction Historical iron can be maintained for years of use and enjoyment provided that some basic care and attention is given to its preservation. The conservation staff of The Henry Ford have compiled the information in this fact sheet to help individuals care for their objects and collections. The first step in the care of collections is to understand and minimize conditions that can cause damage. The second step is to follow basic guidelines for care, handling and cleaning. Please note - this fact sheet will present a brief overview of the care of iron objects, stressing good storage as the best method of preservation. It does not address the serious problems of preserving archaeological metals excavated from land or marine sites. People who collect Un conserved archaeological artifacts should be aware that those types of objects are rarely stable if left untreated and require significant specialist intervention. Please contact a conservator if you need assistance with conservation of these materials. Iron is a common metal in historical collections. It is found in a variety of alloys, known as "ferrous metals", including wrought iron, cast iron and steel. Galvanized or tin-plated sheet is also a familiar material in historical collections. Ferrous metals are magnetic so the presence of iron can, therefore, be easily identified with the use of a magnet. Types of Damage Poor handling and inappropriate storage are the major causes of damage to iron artifacts and can result in corrosion and physical damage to the object. -

Iron, Steel and Swords Script
Iron Ores General Remarks This planet consists of iron (32.1%), oxygen (30.1%), silicon (15.1%), magnesium (13.9%), sulfur (2.9%), nickel (1.8%), calcium (1.5%), and aluminium (1.4%); the remaining 1.2% are "trace amounts" of the 80 or so remaining elements. Most of that iron constitutes the core of the planet but we will not run out of iron compounds or iron ore found near to the surface for some time to come. Why is iron so prominent in this (and other) planet ? Because planets were formed from the stuff bred inside the first stars. The reaction chain for generating energy by nuclear fusion starts with hydrogen and stops after iron has been bred. Iron, if you like, is the ash eventually produced by fusing hydrogen to helium, helium and hydrogen to lithium, and so on. When those first stars "burnt out" after a few billon years, some of them coughed up their ashes in a mighty supernova explosion. In time, new stars were formed. Some of these are still burning and visible at night. One (our very own sund) is only visible during the day from a clumped-together iron-rich ash ball called earth. Advanced From the viewpoint of chemistry, iron (like copper) is a tricky element that can form many oxides, sulfides, carbonates, and so on. All these compounds could be used for smelting iron but some are better for that purpose than others. Sulfides are usually bad news. We know that from smelting copper and expect similar problems when smelting iron. -

Iron Making in Argyll
HISTORIC ARGYLL 2009 Iron smelting in Argyll, and the chemistry of the process by Julian Overnell, Kilmore. The history of local iron making, particularly the history of the Bonawe Furnace, is well described in the Historic Scotland publication of Tabraham (2008), and references therein. However, the chemistry of the processes used is not well described, and I hope to redress this here. The iron age started near the beginning of the first millennium BC, and by the end of the first millennium BC sufficient iron was being produced to equip thousands of troops in the Roman army with swords, spears, shovels etc. By then, local iron making was widely distributed around the globe; both local wood for making charcoal, and (in contrast to bronze making) local sources of ore were widely available. The most widely distributed ore was “bog iron ore”. This ore was, and still is, being produced in the following way: Vegetation in stagnant water starts to rot and soon uses up all the available oxygen, and so produces a water at the bottom with no oxygen (anaerobic) which also contains dissolved organic compounds. This solution is capable of slowly leaching iron from the soils beneath to produce a weak solution of dissolved iron (iron in the reduced, ferrous form). Under favourable conditions this solution percolates down until it meets some oxygen (or oxygenated water) at which point the iron precipitates (as the oxidized ferric form). This is bog iron ore and comprises a somewhat ill-defined brown compound called limonite (FeOOH.nH2O), often still associated with particles of sand etc. -

The Iron-Ore Resources of Europe
DEPARTMENT OF THE INTERIOR ALBERT B. FALL, Secretary UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, Director Bulletin 706 THE IRON-ORE RESOURCES OF EUROPE BY MAX ROESLER WASHINGTON GOVERNMENT PRINTING OFFICE 1921 CONTENTS. Page. Preface, by J. B. Umpleby................................................. 9 Introduction.............................................................. 11 Object and scope of report............................................. 11 Limitations of the work............................................... 11 Definitions.........................:................................. 12 Geology of iron-ore deposits............................................ 13 The utilization of iron ores............................................ 15 Acknowledgments...................................................... 16 Summary................................................................ 17 Geographic distribution of iron-ore deposits within the countries of new E urope............................................................. 17 Geologic distribution................................................... 22 Production and consumption.......................................... 25 Comparison of continents.............................................. 29 Spain..................................................................... 31 Distribution, character, and extent of the deposits....................... 31 Cantabrian Cordillera............................................. 31 The Pyrenees.................................................... -

The Iron Ores of Maryland, with an Account of the Iron Industry
Hass 77,>0 3- Book ffjZd6 MARYLAND GEOLOGICAL AND ECONOMIC SURVEY WM. BULLOCK CLARK, State Geologist REPORT ON THE IRON ORES OF MARYLAND WITH AN ACCOUNT OF THE IRON INDUSTRY BY JOSEPH T. SINGEWALD, JR.- (Special Publication, Volume IX, Part III) THE JOHNS HOPKINS PRESS Baltimore, December, 1911 / / MARYLAND GEOLOGICAL AND ECONOMIC SURVEY WM. BULLOCK CLARK, State Geologist REPORT ON r? Sr THE IRON ORES OF MARYLAND / £ WITH AN ACCOUNT OF THE IRON INDUSTRY BY JOSEPH T. SINGEWALD, JR. M (Special Publication, Volume IX, Part III) THE JOHNS HOPKINS PRESS • Baltimore, December, 1911 V n, ffi ft- sre so i CONTENTS PAGE PART III. REPORT ON THE IRON ORES OF MARYLAND, WITH AN ACCOUNT OF THE IRON INDUSTRY. By Joseph T. Sxngewald, Jr. 121 The Ores of Iron.123 Magnetite . 124 Hematite . 124 Limonite . 124 Carbonate or Siderite. 125 Impurities in the Ores and Their Effects. 125 Mechanical Impurities. 125 Chemical Impurities. 126 Practical Considerations. 127 History of the Maryland Iron Industry. 128 The Colonial Period. 128 The Period from 1780 to 1830. 133 • The Period from 1830 to 1885. 133 The Period from 1885 to the present time. 136 Description of Maryland Iron Works.139 Maryland Furnaces. 139 Garrett County..'... 139 Allegany County. 139 Washington County. 143 Frederick County. 146 Carroll County. 149 Baltimore County. 150 Baltimore City. 159 Harford County. 160 Cecil County. 162 Howard County. 168 Anne Arundel County. 169 Prince George’s County. 171 Worcester County. 172 9 Other Iron Works in Maryland. 173 Allegany County. 173 Baltimore County. 173 Cecil County. 174 CONTENTS PAGE Queen Anne’s County.