The Production of High Carbon Steel Directly in Bloomery Process: Theoretical Bases and Metallographic Analyses of the Experiments Results
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Today's Challenges. Tomorrow's Opportunities
See page 215 for more information. Today’s Challenges. Tomorrow’s Opportunities. The World’s Largest Annual Steel Conference and Exposition TECHNICAL CONFERENCE 550+ Presentations Learn about cutting-edge processes and technological advancements that power today’s progressive industry. Plant Tours See the latest technology and industry processes up close with tours of: • ArcelorMittal Cleveland SOLD OUT • Charter Steel – Cleveland • TimkenSteel Corp. – Faircrest Plant Plant tours typically sell out, so reserve your spot early. REGISTRATION Full Conference Member US$650 Non-Member US$850* Register by 13 April 2015 and save up to US$100 on One-Day Conference Member US$475 Non-Member US$675* full conference or one-day registration. Exposition Only Member FREE Non-Member US$50 *Includes AIST membership AISTech.org EXPOSITION 500+ Exhibitors With 245,000 sq. ft. (22,760 m2) of exhibit space, AISTech 2015 is your opportunity to develop your contacts and promote your business with the individuals who specify, purchase, design and operate a variety of plants and facilities all over the world. Contact [email protected] to reserve your exhibit space and sponsorships today. Lodging AIST has reserved a block of rooms at several hotels in downtown Cleveland. We strongly encourage you to reserve your hotel room well in advance. The block sells out quickly! Reserve your room today at AISTech.org. NETWORK 8,000 Global Industry Professionals Strengthen your network by interacting with your steel industry peers during AISTech’s numerous events, programs and exposition. 550+ Presentations Visas Learn about cutting-edge processes and technological AIST provides letters of invitation to registered international advancements that power today’s progressive industry. -

STUFF MATTERS Hold Together Our Physical World
Watch author photo color. Use CMYK file provided for target. Color substitutions for C,M,Y channels. PMS 801 C for C / PMS 806 C for M / PMS 803 C for Y Process Black / PMS 185 C Red — Scuff Free Matte Lamination $26.00 Higher in Canada MARK MIODOWNIK An eye-opening adventure deep inside the everyday materials that surround us, packed with “I stayed up all night reading this book. Miodownik writes with such knowledge, such enthusiasm, such a palpable love for his subject.” surprising stories and fascinating science — OLIVER SACKS, author of Hallucinations Why is glass see-through? What makes elastic stretchy? Why does a paper clip bend? Why does any “Concrete, chocolate, paper, porcelain; this is a fascinating and informative MARK MIODOWNIK material look and behave the way it does? These are account of the ‘stuff’ of our everyday lives.” the sorts of questions that Mark Miodownik is con- — PENNY LE COUTEUR, coauthor of Napoleon’s Buttons: stantly asking himself. A globally renowned materials How 17 Molecules Changed History scientist, Miodownik has spent his life exploring ob- jects as ordinary as an envelope and as unexpected as concrete cloth, uncovering the fascinating secrets that “It is a rare thing for a true scientist to be able to explain how things work so STUFF MATTERS hold together our physical world. clearly to the layperson — and even rarer to do so in such an entertaining fashion. MATTERS STUFF In Stuff Matters, Miodownik entertainingly exam- No one who reads this book will look at the world quite the same again.” ines the materials he encounters in a typical morn- — KATE ASCHER, author of The Works, ing, from the steel in his razor and the graphite in his The Heights, and The Way to Go pencil to the foam in his sneakers and the concrete in a nearby skyscraper. -
Bloomery Design
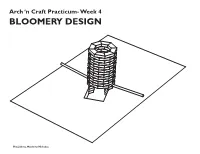
Arch ‘n Craft Practicum- Week 4 BLOOMERY DESIGN Hiu, Julieta, Matthew, Nicholas 7 3/4" The Stack: 3 3/4" In our ideal model we would use refractory bricks, either rectangular bricks or curved bricks which would help us make a circular 1'-8" stack. The stack would be around 3 feet tall with a 1 foot diameter for the inner wall of the stack. There will be openings about 3 brick lengths up(6-9 inches) from the ground on 1'-0" opposite ends of the stack. Attached to these openings will be our tuyeres which in turn will connect to our air sources. Building a circular stack would be beneficial to the type of air distribution we want to create within our furnace; using two angled tuyeres we would like air to move clockwise and counterclock- Bloomery Plan wise. We would like to line the inside of the stack with clay to provide further insulation. Refractory bricks would be an ideal material to use because the minerals they are com- posed of do not change chemically when 1'-0" exposed to high heat, some examples of these materials are aluminum, manganese, silica and zirconium. Other comments: 3'-0" Although we have opted to use refractory bricks, which would be practical for our intended purpose, we briefly considered using Vycor glass which can withstand high tem- peratures of up to 900 degrees celsius or approximately 1652 degrees Fahrenheit, which Tuyere height: Pitched -10from horizontal 7" would be enough for our purposes. Bloomery Section Ore/ Charcoal: Our group has decided to use Magnetite ore along with charcoal for the smelt. -

On a Mathematical Model for Case Hardening of Steel
On a mathematical model for case hardening of steel vorgelegt von Dott.ssa Lucia Panizzi Von der Fakultät II ‚ Mathematik und Naturwissenschaften der Technischen Universität Berlin zur Erlangung des akademischen Grades Doktor der Naturwissenschaften Dr. rer. nat. sowie der Classe di Scienze der Scuola Normale Superiore di Pisa als Diploma di Perfezionamento in Matematica per la Tecnologia e l’Industria genehmigte Dissertation Promotionsausschuss: Berichter/Gutachter: Prof. A. Fasano (Univerisità di Firenze) Berichter/Gutachter: Prof. D. Hömberg (Technische Universität Berlin) Gutachter: Prof. L. Formaggia (Politecnico di Milano) Gutachter: Prof. M. Primicerio (Università di Firenze) Gutachter: Prof. F. Tröltzsch (Technische Universität Berlin) Gutachter: Prof. P. Wittbold (Technische Universität Berlin) Tag der wissenschaftlichen Aussprache: 05.03.2010 Berlin 2010 D83 i Eidesstattliche Versicherung Hiermit erkläre ich, dass die vorliegende Dissertation: On a mathematical model for case hardening of steel selbständig verfasst wurde. Die benutzten Hilfsmittel und Quellen wurden von mir angegeben, weitere wurden nicht verwendet. ii Erklärung Hiermit erkläre ich, dass die Anmeldung meiner Promotionsabsicht früher nicht bei einer anderen Hochschule oder einer anderen Fakultät beantragt wurde. Teile meiner Dissertation sind darüber hinaus schon veröffentlich worden, welche im Folgenden aufgelistet sind: • D. Hömberg, A. Fasano, L. Panizzi A mathematical model for case hardening of steel. angenommen zur Veröffentlichung in "Mathematical Models and Methods in Applied Sciences" (M3AS), 2009. • P. Krejčí, L. Panizzi. Regularity and uniqueness in quasilinear parabolic systems. angenommen zur Veröffentlichung in "Applications of Mathematics", 2009. iii Compendio Nonostante la disponibilità di numerosi nuovi materiali, l’acciaio rimane il ma- teriale di base della moderna società industriale. L’uso dell’ acciaio con peculiari caratteristiche (durezza, resistenza all’uso, malleabilità etc.) è perciò assai diffuso in molti settori della tecnica. -

UNDERSTANDING and MEASURING DECARBURIZATION Understanding the Forces Behind Decarburization Is the First Step Toward Minimizing Its Detrimental Effects
22 UNDERSTANDING AND MEASURING DECARBURIZATION Understanding the forces behind decarburization is the first step toward minimizing its detrimental effects. George F. Vander Voort, FASM*, Struers Inc. (Consultant), Cleveland ecarburization is detrimental to crostructure of carbon contents close to the maximum depth of decarburization the wear life and fatigue life of the core may be difficult to discern. The is established. Because the carbon dif- steel heat-treated components. MAD determined by hardness traverse fusion rate increases with temperature ADVANCED MATERIALS & PROCESSES | FEBRUARY 2015 2015 FEBRUARY | & PROCESSES MATERIALS ADVANCED D This article explores some factors that may be slightly shallower than that de- when the structure is fully austenitic, cause decarburization while concentrat- termined by quantitative carbon analysis MAD also increases as temperature rises ing on its measurement. In most produc- with the electron microprobe. This is es- above the Ac3. For temperatures in the tion tests, light microscopes are used to pecially true when the bulk carbon con- two-phase region, between the Ac1 and scan the surface of a polished and etched tent exceeds about 0.45 wt%, as the rela- Ac3, the process is more complex. Carbon cross-section to find what appears to be tionship between carbon in the austenite diffusion rates in ferrite and austenite the greatest depth of total carbon loss before quenching to form martensite and are different, and are influenced by both (free-ferrite depth, or FFD) and the great- the as-quenched hardness loses its linear temperature and composition. est depth of combined FFD and partial nature above this carbon level. Decarburization is a serious prob- loss of carbon to determine the maxi- lem because surface properties are infe- mum affected depth (MAD). -

An Introduction to Nitriding
01_Nitriding.qxd 9/30/03 9:58 AM Page 1 © 2003 ASM International. All Rights Reserved. www.asminternational.org Practical Nitriding and Ferritic Nitrocarburizing (#06950G) CHAPTER 1 An Introduction to Nitriding THE NITRIDING PROCESS, first developed in the early 1900s, con- tinues to play an important role in many industrial applications. Along with the derivative nitrocarburizing process, nitriding often is used in the manufacture of aircraft, bearings, automotive components, textile machin- ery, and turbine generation systems. Though wrapped in a bit of “alchemi- cal mystery,” it remains the simplest of the case hardening techniques. The secret of the nitriding process is that it does not require a phase change from ferrite to austenite, nor does it require a further change from austenite to martensite. In other words, the steel remains in the ferrite phase (or cementite, depending on alloy composition) during the complete proce- dure. This means that the molecular structure of the ferrite (body-centered cubic, or bcc, lattice) does not change its configuration or grow into the face-centered cubic (fcc) lattice characteristic of austenite, as occurs in more conventional methods such as carburizing. Furthermore, because only free cooling takes place, rather than rapid cooling or quenching, no subsequent transformation from austenite to martensite occurs. Again, there is no molecular size change and, more importantly, no dimensional change, only slight growth due to the volumetric change of the steel sur- face caused by the nitrogen diffusion. What can (and does) produce distor- tion are the induced surface stresses being released by the heat of the process, causing movement in the form of twisting and bending. -

20. Iron and Steel Part II Copy
USES FOR TYPES OF STEELS • Low carbon steel (0.08 - 0.35 % carbon) is ductile with low brittleness. It is is used for ANCIENT IRON auto body parts, home appliances, tin cans, I beams for construction. AND STEEL • Medium carbon steel (0.35 - 0.5 % carbon) (Part II) is used as crankshaft, gears, railroad axels. (Part II) They are difficult to weld. • High carbon steel (> 0.5 % carbon) is used for railroad wheels and rails, wrenches, steel cable, tools, dies, piano wire etc. BLAST FURNACE CAST IRON (PIG IRON) • Contains 1.5 - 5 % carbon. • Its melting point is 1130 oC. • The metal will shatter with a hard blow. • Carbon in the form of graphite exists as flakes • Graphite serves as a lubricant (excellent bearing material). 1 Cast Grey Iron Blast furnace at Cast Grey Iron Karabük Iron works Cast Gray Iron Graphite flakes THE FINERY CAST IRON OBJECTS THE FINERY Cast cannon 15th. Cent. 2 INDIRECT METHOD OF STEEL BESSEMER PROCESS PRODUCTION (THE BESSEMER PROCESS) Crude cast iron from the blast furnace is remelted in a hearth (the finary) and subjected to a forced draught of air. The excess carbon is oxidized to carbon dioxide. The refined pig iron which is called malleable iron is now ready for final forging. BESSEMER PROCESS FORMS OF IRON CAST IRON Decarburization From Blast Furnace M.P. 1130oC 1,5-4.5 % C Bessemer STEEL Process M.P. 1400o C 0.1-0.9 % C WROUGHT IRON From Bloomery Cementation M.P 1535o C 0.06 % C Carburization 3 DIFFERENCES BETWEEN BLOOMERY ALLOY STEELS AND BLAST FURNACE • Increase in temperature (1300 - 1400oC) • Alloy steel describes steel that contain one or more alloying elements in addition to carbon. -

AISI | Electric Arc Furnace Steelmaking
http://www.steel.org/AM/Template.cfm?Section=Articles3&TEMPLATE=/CM/HTMLDisplay.cfm&CONTENTID=12308 Home Steelworks Home Electric Arc Furnace Steelmaking By Jeremy A. T. Jones, Nupro Corporation SIGN UP to receive AISI's FREE e-news! Read the latest. Email: Name: Join Courtesy of Mannesmann Demag Corp. FURNACE OPERATIONS The electric arc furnace operates as a batch melting process producing batches of molten steel known "heats". The electric arc furnace operating cycle is called the tap-to-tap cycle and is made up of the following operations: Furnace charging Melting Refining De-slagging Tapping Furnace turn-around Modern operations aim for a tap-to-tap time of less than 60 minutes. Some twin shell furnace operations are achieving tap-to-tap times of 35 to 40 minutes. 10/3/2008 9:36 AM http://www.steel.org/AM/Template.cfm?Section=Articles3&TEMPLATE=/CM/HTMLDisplay.cfm&CONTENTID=12308 Furnace Charging The first step in the production of any heat is to select the grade of steel to be made. Usually a schedule is developed prior to each production shift. Thus the melter will know in advance the schedule for his shift. The scrap yard operator will prepare buckets of scrap according to the needs of the melter. Preparation of the charge bucket is an important operation, not only to ensure proper melt-in chemistry but also to ensure good melting conditions. The scrap must be layered in the bucket according to size and density to promote the rapid formation of a liquid pool of steel in the hearth while providing protection for the sidewalls and roof from electric arc radiation. -

Parallel Deformation of the Metals
Modeling and Numerical Simulation of Material Science, 2013, 3, 79-83 http://dx.doi.org/10.4236/mnsms.2013.33010 Published Online July 2013 (http://www.scirp.org/journal/mnsms) Parallel Deformation of the Metals Chetan Nikhare Mechanical Engineering Department, The Pennsylvania State University, Erie, USA Email: [email protected] Received January 30, 2013; revised March 3, 2013; accepted March 15, 2013 Copyright © 2013 Chetan Nikhare. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The metal goes into the plastic deformation after the application of external load. Most of the metal forming industries work on this principle of plastic deformation. Thus the understanding of plastic deformation in the metal forming indus- try is important. The research on the single material plastic deformation has been carried out from many centuries be- fore the era of Tresca. In this study the two metals 0.05% C steel annealed (soft metal) and 0.6% C steel quenched and tempered (hard metal) were deformed plastically in the parallel combination in the composite form. This study has been carried out with simple mathematical theory and simulated numerical model. The comparison shows the exact match between the mathematical and numerical results. It is also observed that the individual metal thickness affects the de- formation flow curve. Keywords: Plasticity; Bi-Metal; Metal Forming; Mathematical Model; Numerical Model 1. Introduction at soda fountain corner through the straw which was long enough from her mouth reach. -

Analysis of Scale-Up of a Shaft Furnace by Process Engineering-From the Iron-Manufacturing Experiment by Using Bei-Tetsu in Hippo Tatara
ISIJ International, Vol. 54 (2014), No. 5, pp. 1051–1058 Analysis of Scale-up of a Shaft Furnace by Process Engineering - From the Iron-manufacturing Experiment by Using Bei-tetsu in Hippo Tatara - Yoshiyuki MATSUI,1)* Keiichi TERASHIMA2) and Reijiro TAKAHASHI3) 1) Kobelco Research Institute, Inc., 1-5-5, Takatsukadai, Nishi-ku, Kobe, Japan. 2) Chiba Institute of Technology, 2-17-1, Tsudanuma, Narashino, Chiba, Japan. 3) Former Tohoku University, 2-1-1, Katahira, Aoba-ku, Sendai, Miyagi, Japan. (Received on November 30, 2013; accepted on March 3, 2014) “Iron technology and history forum” obtained the opportunity to participate in the 38th iron-manufacture experiment by using Bei-tetsu (lump iron ore with rice cake shape) conducted like in 1859 (Ansei era 6) by “the committee of restoring iron manufacture of “Hippo” (Village of Marumori-Town, Igu-County, Miyagi-Prefecture, Japan)” as early reconstruction assistance from the Great East Japan Earthquake in 2011. This experimental result was analyzed by process engineering and discovered the bird’s eye view on the conversion from the Japanese-style indirect iron-making into the shaft furnace historically and tech- nically. KEY WORDS: Tatara; shaft furnace; Blast Furnace; similarity rule; golden ratio. the old Sendai-feudal domain. 1. Introduction By restoring northeastern industrial history by carrying It is said that “Technology is sublimated to learning as it out using the Bei-tetsu from Taneyama plateau of the north- grows on a large scale. This is certain by theoretic common ernmost tip of old Sendai-feudal domain as the tatara mate- evolution.” In manufacturing technique, the blast furnace is rial of the Marumori-tawn renaissance at the southernmost the maximum scale serves as the comprehensive tip of the old Sendai-feudal domain as traditional craftsman- engineering1) of many learning fields as well as rolling. -

CONTROL of DECARBURIZATION of STEEL Paul Shefsiek
CONTROL OF DECARBURIZATION OF STEEL Paul Shefsiek Introduction Historically, heating steel for forming, forging or rolling, was done in electrical resistance or natural gas heated furnaces. It was inevitable that these furnaces contained Oxygen and Decarburization of the steel surface occurred. This decarburization was either ignored or minimized by coating the steel with “ stopoff “. Also, this decarburization was minimized through the use nitrogen atmosphere furnaces. But, decarburization could not be reduced to acceptable limits until the Chemical Potential of the Carbon in the furnace atmosphere matched the dissolved Carbon in the steel. The Furnace Industry that provides Equipment for the processing of steel, because of the temperature ranges involved, divided itself into two categories, namely, Reheat and Heat Treating. Each has its own special Technology. But, because of this division, quite often one division does not know the Technology of the other. In some cases this has been unfortunate. If the Reheat side of the Industry had the Carburizing Technology of the Heat Treating side, this Technology could have been applied to applications where preventing Decarburizing was a requirement. Therefore, the purpose of the following discussion is to separate out that part of Carburizing Technology that is applicable to preventing Decarburization in Reheat applications. Fundamentals Control of the Decarburization of Steel has been standardized to the monitoring of - The Metal Temperature (Furnace Temperature) - The Atmosphere Concentration of Carbon Monoxide (CO) - The Atmosphere Concentration of Carbon Dioxide (CO2). Knowing the values of these three (3) parameters and the knowledge of - Saturated Austenite in Iron - The Alloy Content of the Steel - The Equilibrium Constant for the Controllable Chemical Reaction between the Steel and the Gas Atmosphere it can be determined if the Atmosphere will prevent Decarburization of the Steel. -

" Tatara" Process*
" Tatara" Process* - A Pig Iron- and Steel-Making Process, Transmitted from Ancient Times in Japan - By lukichi KOZ lIka** Sy n op sis at " Cha-no-Yu ") were m a d e from it. From Gncient times 111 j ajJa n Ihere are excellent swords called In the following the a uthor d escribes " T a tara" " NijJjJon- t6" as a symbol of " Samurai" . The)' are weLL-knowll and p rocess putting the stress on the direct steelmaking sland high in their aesthetic value aLL over Ihe world. The material of m ethod, " Keraoshi " . " N ijJjJo /l-to" is " Tamahagallc" (c/'ltde steel ) or " H ocho-Ietsu" (c rude wrought iron) produced by " T atara " process which was devel II. The Historical Development of "Tatara )) oped jJeCl.tiiariy ill j ajJa Il alld has bem hallded down to us f rom ollr Process ancestors. This pajJer briefly describes the method of " Totaro " /J I"Qcess and From the a rchaeological viewpoint, it is regarded several malters related to it. that the process was brough t to j a pan for the firsL Lime from the Asia tic Con tinent in the first a nd second I. Introduction centuries. The primitive m ethod of the process ex R aw m ateria l used in " T atara " process is thc isted in the fa bulous age of j a pa n, when our a ncestors. specia l iron sand ca lled " M asa " or "Aka m e" whi ch manufactured by aid of natura l draft a mixture of is mined only in San-in distri ct, sou th-wes t pa rt of iron, steel, sponge iron, slag, a nd charcoal in a hole the m a in isla nd of j apa n .