7313200007 DEC Permit Conditions FINAL Page 1 PERMIT Under The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Crucible Industries
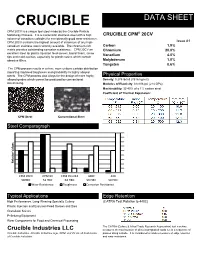
CRUCIBLE DATA SHEET CPM 20CV is a unique tool steel made by the Crucible Particle ® Metallurgy Process. It is a martensitic stainless steel with a high CRUCIBLE CPM 20CV volume of vanadium carbides for exceptionally good wear resistance. CPM 20CV contains the highest amount of chromium of any high- Issue #1 vanadium stainless steel currently available. The chromium rich Carbon 1.9% matrix provides outstanding corrosion resistance. CPM 20CV an Chromium 20.0% excellent steel for plastic injection feed screws, barrel liners, screw Vanadium 4.0% tips and mold cavities, especially for plastic resins which contain abrasive fillers. Molybdenum 1.0% Tungsten 0.6% The CPM process results in a finer, more uniform carbide distribution imparting improved toughness and grindability to highly alloyed steels. The CPM process also alloys for the design of more highly Physical Properties alloyed grades which cannot be produced by conventional Density: 0.275 lb/in3 (7616 kg/m3) steelmaking. Modulus of Elasticity: 31x106 psi (214 GPa) Machinability: 35-40% of a 1% carbon steel Coefficient of Thermal Expansion: CPM Steel Conventional Steel Steel Comparagraph Relative Relative Values CPM 20CV CPM 9V CPM Rex M4 440C 420 58HRC 53 HRC 63 HRC 56 HRC 50 HRC Wear Resistance Toughness Corrosion Resistance Typical Applications Edge Retention (CATRA Test Relative to 440C) High Performance, Long-Wearing Specialty Cutlery (CATRA Test Relative to 440C) Plastic Injection and Extrusion Feed Screws and Dies Granulator Knives Pelletizing Equipment Wear Components for Food and Chemical Processing The CATRA (Cutlery & Allied Trade Research Association) test machine Crucible Industries LLC measures the total number of silica impregnated cards cut in a sequence of Crucible Industries, Crucible Industries logo, CPM, and 9V are all trademarks passes along a blade. -

Improved Processing Techniques for Inclusion-Free Steel for Bearing and Mechanical Component Applications
Symposium: 12th International Symposium on Rolling Bearing Steels – Progress in Bearing Steel Metallurgical Testing and Quality Assurance Improved Processing Techniques for Inclusion-Free Steel for Bearing and Mechanical Component Applications Christopher DellaCorte1 ABSTRACT High-hardness, high-carbide powder metallurgy tool steels such as M62 enable the use of ball bearings at extremely high load and stress levels. Operation under such conditions increases the potential for rolling contact fatigue failure attributed to nonmetallic inclusions. To address this challenge, industry has sought ever-cleaner steels, but the results have been uneven, owing to the random nature of the occurrence of such material flaws. Careful melting processes and thorough ingot inspections prior to bearing manufacture are common approaches to avoid inclusions. Selecting only the cleanest portions of an ingot may result in bearings relatively free from material flaws. This approach is not always successful because detrimental flaws that exist deep within an ingot can pass inspections undetected, potentially causing subsequent failure. Recent efforts to commercialize an intermetallic material, 60NiTi, for rolling element bearings demonstrates a pathway to produce bearing steel that is free from unwanted ceramic particle inclusions. In this paper, the process used to make bearing-grade inclusion-free NiTi alloys is 1 NASA Glenn Research Center, Cleveland, Ohio, 44135, USA; ORCID (Note: Go to www.orcid.org for details) Page 1 of 30 described and applied to steelmaking. At its core, the NiTi process differs from steelmaking in one key aspect. NiTi alloys are made from elementally pure starting materials that are melted, blended, and processed in equipment that minimizes exposure to oxygen and other sources of contamination, ensuring an inclusion-free product. -

On a Mathematical Model for Case Hardening of Steel
On a mathematical model for case hardening of steel vorgelegt von Dott.ssa Lucia Panizzi Von der Fakultät II ‚ Mathematik und Naturwissenschaften der Technischen Universität Berlin zur Erlangung des akademischen Grades Doktor der Naturwissenschaften Dr. rer. nat. sowie der Classe di Scienze der Scuola Normale Superiore di Pisa als Diploma di Perfezionamento in Matematica per la Tecnologia e l’Industria genehmigte Dissertation Promotionsausschuss: Berichter/Gutachter: Prof. A. Fasano (Univerisità di Firenze) Berichter/Gutachter: Prof. D. Hömberg (Technische Universität Berlin) Gutachter: Prof. L. Formaggia (Politecnico di Milano) Gutachter: Prof. M. Primicerio (Università di Firenze) Gutachter: Prof. F. Tröltzsch (Technische Universität Berlin) Gutachter: Prof. P. Wittbold (Technische Universität Berlin) Tag der wissenschaftlichen Aussprache: 05.03.2010 Berlin 2010 D83 i Eidesstattliche Versicherung Hiermit erkläre ich, dass die vorliegende Dissertation: On a mathematical model for case hardening of steel selbständig verfasst wurde. Die benutzten Hilfsmittel und Quellen wurden von mir angegeben, weitere wurden nicht verwendet. ii Erklärung Hiermit erkläre ich, dass die Anmeldung meiner Promotionsabsicht früher nicht bei einer anderen Hochschule oder einer anderen Fakultät beantragt wurde. Teile meiner Dissertation sind darüber hinaus schon veröffentlich worden, welche im Folgenden aufgelistet sind: • D. Hömberg, A. Fasano, L. Panizzi A mathematical model for case hardening of steel. angenommen zur Veröffentlichung in "Mathematical Models and Methods in Applied Sciences" (M3AS), 2009. • P. Krejčí, L. Panizzi. Regularity and uniqueness in quasilinear parabolic systems. angenommen zur Veröffentlichung in "Applications of Mathematics", 2009. iii Compendio Nonostante la disponibilità di numerosi nuovi materiali, l’acciaio rimane il ma- teriale di base della moderna società industriale. L’uso dell’ acciaio con peculiari caratteristiche (durezza, resistenza all’uso, malleabilità etc.) è perciò assai diffuso in molti settori della tecnica. -

UNDERSTANDING and MEASURING DECARBURIZATION Understanding the Forces Behind Decarburization Is the First Step Toward Minimizing Its Detrimental Effects
22 UNDERSTANDING AND MEASURING DECARBURIZATION Understanding the forces behind decarburization is the first step toward minimizing its detrimental effects. George F. Vander Voort, FASM*, Struers Inc. (Consultant), Cleveland ecarburization is detrimental to crostructure of carbon contents close to the maximum depth of decarburization the wear life and fatigue life of the core may be difficult to discern. The is established. Because the carbon dif- steel heat-treated components. MAD determined by hardness traverse fusion rate increases with temperature ADVANCED MATERIALS & PROCESSES | FEBRUARY 2015 2015 FEBRUARY | & PROCESSES MATERIALS ADVANCED D This article explores some factors that may be slightly shallower than that de- when the structure is fully austenitic, cause decarburization while concentrat- termined by quantitative carbon analysis MAD also increases as temperature rises ing on its measurement. In most produc- with the electron microprobe. This is es- above the Ac3. For temperatures in the tion tests, light microscopes are used to pecially true when the bulk carbon con- two-phase region, between the Ac1 and scan the surface of a polished and etched tent exceeds about 0.45 wt%, as the rela- Ac3, the process is more complex. Carbon cross-section to find what appears to be tionship between carbon in the austenite diffusion rates in ferrite and austenite the greatest depth of total carbon loss before quenching to form martensite and are different, and are influenced by both (free-ferrite depth, or FFD) and the great- the as-quenched hardness loses its linear temperature and composition. est depth of combined FFD and partial nature above this carbon level. Decarburization is a serious prob- loss of carbon to determine the maxi- lem because surface properties are infe- mum affected depth (MAD). -

Table of Contents
Table of Contents INTRODUCTION…………………………………………………………………………………………………. 3 SUNY-ESF FIRST DESTINATION SURVEY: SUMMARY REPORT FOR 2016……………………... 7 Overall Responses…………………………………………………………………………………………. 7 Employment Information……………………..…………………………………………………………… 8 Continuing Education Information……………..…………………………………………………………. 11 Participation In University Programs…………..………………………………………………………….. 11 DEPARTMENT OF CHEMISTRY…………………..………………………………………………….……... 12 Overall Responses………………………………..…………………………………………………….….. 12 Employment Information………………………..………………………………………………………… 12 Continuing Education Information……………..…………………………………………………………. 14 Participation in University Programs……………..……………………………………………………….. 14 DEPARTMENT OF ENVIRONMENTAL and FOREST BIOLOGY……………………………..………. 15 Overall Responses…………………………………………………………………………………………. 15 Employment Information…………………………………………………………………….……………. 15 Continuing Education Information…………………………………………………………….………….. 21 Participation in University Programs……………………………………………………………………… 22 DEPARTMENT OF ENVIRONMENTAL RESOURCES ENGINEERING……………………..……….. 23 Overall Responses………………………………………………………………………………………….. 23 Employment Information…………………………………………………………………………………... 23 Continuing Education Information…………………………………………………………………………. 26 Participation in University Programs………………………………………………………………………. 27 DEPARTMENT OF ENVIRONMENTAL SCIENCE……………………………..………............................. 28 Overall Responses………………………………………………………………………………………….. 28 Employment Information………………………………………………………………………………….. 28 Continuing Education -

An Introduction to Nitriding
01_Nitriding.qxd 9/30/03 9:58 AM Page 1 © 2003 ASM International. All Rights Reserved. www.asminternational.org Practical Nitriding and Ferritic Nitrocarburizing (#06950G) CHAPTER 1 An Introduction to Nitriding THE NITRIDING PROCESS, first developed in the early 1900s, con- tinues to play an important role in many industrial applications. Along with the derivative nitrocarburizing process, nitriding often is used in the manufacture of aircraft, bearings, automotive components, textile machin- ery, and turbine generation systems. Though wrapped in a bit of “alchemi- cal mystery,” it remains the simplest of the case hardening techniques. The secret of the nitriding process is that it does not require a phase change from ferrite to austenite, nor does it require a further change from austenite to martensite. In other words, the steel remains in the ferrite phase (or cementite, depending on alloy composition) during the complete proce- dure. This means that the molecular structure of the ferrite (body-centered cubic, or bcc, lattice) does not change its configuration or grow into the face-centered cubic (fcc) lattice characteristic of austenite, as occurs in more conventional methods such as carburizing. Furthermore, because only free cooling takes place, rather than rapid cooling or quenching, no subsequent transformation from austenite to martensite occurs. Again, there is no molecular size change and, more importantly, no dimensional change, only slight growth due to the volumetric change of the steel sur- face caused by the nitrogen diffusion. What can (and does) produce distor- tion are the induced surface stresses being released by the heat of the process, causing movement in the form of twisting and bending. -

Annual Report of the Director Bureau of Standards to the Secretary Of
ANNUAL REPORT OF THE DIRECTOR BUREAU OF STANDARDS TO THE SECRETARY OF COMMERCE FOR THE FISCAL YEAR ENDED JUNE 30, 1915 WASHINGTON GOVERNMENT PRINTING OFFICE 1915 ANNUAL REPORT OF THE DIRECTOR BUREAU OF STANDARDS TO THE SECRETARY OF COMMERCE FOR THE FISCAL YEAR ENDED JUNE 30, 1915 WASHINGTON GOVERNMENT PRINTING OFFICE 1915 CONTENTS. Page. I. Functions, organization, and location 11 1. Standards of measurement 11 2. Pliysical constants 12 3. Standards of quality 12 4. Standards of performance 13 5. Standards of practice 14 6. Relation of the Bureau's work to the public 14 7. Relation of the Bureau's work to the Government service 16 8. Organization 17 9. Location ^ 18 II. Scientific and technical divisions 19 1. Weights and measures 19 Weights and balances 19 Capacity and length measures 19 Sieve testing 20 Volumetric glassware 20 Density tables for petroleum oils 20 Barometry 20 Testing of watches 21 Precision and empirical equations 22 Inspecting and testing scales 22 Operation of test-car equipment 23 Condition of track scales 23 Linear expansion of materials 24 Annual conference on weights and measures 25 Tolerances and specifications 25 So-called net-weight amendment to the pure-food law 26 Standard-barrel act 26 Information furnished on subjects pertaining to measures of length, mass, capacity, and time 26 Publications on weights and measures 27 2. Thermometry, pyrometry, and heat measurements 27 Platinum resistance thermometers 27 A AVheatstone bridge for resistance thermometry 28 A multiple junction thermoelectric thermometer 28 -

20. Iron and Steel Part II Copy
USES FOR TYPES OF STEELS • Low carbon steel (0.08 - 0.35 % carbon) is ductile with low brittleness. It is is used for ANCIENT IRON auto body parts, home appliances, tin cans, I beams for construction. AND STEEL • Medium carbon steel (0.35 - 0.5 % carbon) (Part II) is used as crankshaft, gears, railroad axels. (Part II) They are difficult to weld. • High carbon steel (> 0.5 % carbon) is used for railroad wheels and rails, wrenches, steel cable, tools, dies, piano wire etc. BLAST FURNACE CAST IRON (PIG IRON) • Contains 1.5 - 5 % carbon. • Its melting point is 1130 oC. • The metal will shatter with a hard blow. • Carbon in the form of graphite exists as flakes • Graphite serves as a lubricant (excellent bearing material). 1 Cast Grey Iron Blast furnace at Cast Grey Iron Karabük Iron works Cast Gray Iron Graphite flakes THE FINERY CAST IRON OBJECTS THE FINERY Cast cannon 15th. Cent. 2 INDIRECT METHOD OF STEEL BESSEMER PROCESS PRODUCTION (THE BESSEMER PROCESS) Crude cast iron from the blast furnace is remelted in a hearth (the finary) and subjected to a forced draught of air. The excess carbon is oxidized to carbon dioxide. The refined pig iron which is called malleable iron is now ready for final forging. BESSEMER PROCESS FORMS OF IRON CAST IRON Decarburization From Blast Furnace M.P. 1130oC 1,5-4.5 % C Bessemer STEEL Process M.P. 1400o C 0.1-0.9 % C WROUGHT IRON From Bloomery Cementation M.P 1535o C 0.06 % C Carburization 3 DIFFERENCES BETWEEN BLOOMERY ALLOY STEELS AND BLAST FURNACE • Increase in temperature (1300 - 1400oC) • Alloy steel describes steel that contain one or more alloying elements in addition to carbon. -

CPM 20CV Powder Metal Stainless Tool Steel
DATA SHEET Issue 1 ® CPM 20CV Powder Metal Stainless Tool Steel LATROBE SPECIALTY STEEL your source for all Crucible CPM products Typical Composition C Mn Si Cr W Mo V 1.90 0.30 0.30 20.00 0.60 1.00 4.00 CPM 20CV is a highly wear resistant, powder metallurgy stainless tool steel. The steel contains a large volume of extremely hard vanadium carbides, which provide excellent wear resistance. CPM 20CV contains the highest amount of chromium of any high-vanadium stainless steel currently available. The chromium-rich matrix provides outstanding corrosion resistance. CPM 20CV is a versatile stainless tool steel with a unique combination of high wear resistance, high corrosion resistance, good impact toughness, and excellent polishability. This combination of properties is the result of the fine grain size, small carbides, and superior cleanliness of the powder metallurgy (PM) microstructure. CPM 20CV excels in applications, which require a combination of high wear resistance and high corrosion resistance. The unique combination of properties makes CPM 20CV an excellent steel for plastic injection feed screws, barrel liners, screw tips, and mold cavities, especially for plastic resins which contain abrasive fillers. Other applications include food processing equipment, pelletizer knives, granulator knives, and high- performance custom knives. Relative Properties Toughness Wear Resistance Corrosion Resistance CPM 20CV CPM 9V CPM Rex M4 440C 420 58 HRC 53 HRC 63 HRC 56 HRC 50 HRC Physical Properties Edge Retention (CATRA Test Relative to 440C) -

AISI | Electric Arc Furnace Steelmaking
http://www.steel.org/AM/Template.cfm?Section=Articles3&TEMPLATE=/CM/HTMLDisplay.cfm&CONTENTID=12308 Home Steelworks Home Electric Arc Furnace Steelmaking By Jeremy A. T. Jones, Nupro Corporation SIGN UP to receive AISI's FREE e-news! Read the latest. Email: Name: Join Courtesy of Mannesmann Demag Corp. FURNACE OPERATIONS The electric arc furnace operates as a batch melting process producing batches of molten steel known "heats". The electric arc furnace operating cycle is called the tap-to-tap cycle and is made up of the following operations: Furnace charging Melting Refining De-slagging Tapping Furnace turn-around Modern operations aim for a tap-to-tap time of less than 60 minutes. Some twin shell furnace operations are achieving tap-to-tap times of 35 to 40 minutes. 10/3/2008 9:36 AM http://www.steel.org/AM/Template.cfm?Section=Articles3&TEMPLATE=/CM/HTMLDisplay.cfm&CONTENTID=12308 Furnace Charging The first step in the production of any heat is to select the grade of steel to be made. Usually a schedule is developed prior to each production shift. Thus the melter will know in advance the schedule for his shift. The scrap yard operator will prepare buckets of scrap according to the needs of the melter. Preparation of the charge bucket is an important operation, not only to ensure proper melt-in chemistry but also to ensure good melting conditions. The scrap must be layered in the bucket according to size and density to promote the rapid formation of a liquid pool of steel in the hearth while providing protection for the sidewalls and roof from electric arc radiation. -

CPM S60V Data Sheet
® DATA SHEET CPM S60V Typical Composition C Cr Mo V 2.15 17.0 .4 5.5 CPM S60V is a corrosion resistant, highly wear-resistant steel made by the Crucible Particle Metallurgy process. It is a martensitic stainless steel with a high volume of chromium and vanadium carbides for exceptionally good wear and corrosion resistance. S60V offers substantial improvements in wear resistance over 440C and D2, and other high chromium tool steels, with corrosion resistance much better than 440C. The CPM process results in a finer, more uniform carbide distribution imparting improved toughness and grind ability to high alloy steels. The CPM process also allows the design of more highly alloyed grades which cannot be produced by conventional steelmaking. Typical Applications: Plastic injection and extrusion feed screws, non-return valve components, pelletizing equipment, gate and nozzle inserts, industrial knives, slitters, and cutters, long-wearing specialty cutlery, injection molds and inserts, wear components for food and chemical processing, bearings, bushings, valves, rolls and gear pumps. Special Note to Knifemakers: The recommended hardness for blades is HRC 56/58, which is designed to provide an excellent combination of corrosion resistance, wear resistance and edge toughness. Mechanical Properties Hardness (1) Impact Toughness ft-lbs HRC Heat Treatment CPM S60V 56 (A) 16 CPM S60V 59 (B) 12 CPM S60V 60.5 (C) 11 1.) A=Hardened 1850°F (1010°C), double tempered 400°F (205°C). B=Hardened 1950°F (1065°C), double tempered 400°F (205°C). C=Hardened 2050°F (1120°C), double tempered 400°F (205°C). CONVENTIONAL STEEL CPM STEEL This data sheet is for informational purposes only. -

CONTROL of DECARBURIZATION of STEEL Paul Shefsiek
CONTROL OF DECARBURIZATION OF STEEL Paul Shefsiek Introduction Historically, heating steel for forming, forging or rolling, was done in electrical resistance or natural gas heated furnaces. It was inevitable that these furnaces contained Oxygen and Decarburization of the steel surface occurred. This decarburization was either ignored or minimized by coating the steel with “ stopoff “. Also, this decarburization was minimized through the use nitrogen atmosphere furnaces. But, decarburization could not be reduced to acceptable limits until the Chemical Potential of the Carbon in the furnace atmosphere matched the dissolved Carbon in the steel. The Furnace Industry that provides Equipment for the processing of steel, because of the temperature ranges involved, divided itself into two categories, namely, Reheat and Heat Treating. Each has its own special Technology. But, because of this division, quite often one division does not know the Technology of the other. In some cases this has been unfortunate. If the Reheat side of the Industry had the Carburizing Technology of the Heat Treating side, this Technology could have been applied to applications where preventing Decarburizing was a requirement. Therefore, the purpose of the following discussion is to separate out that part of Carburizing Technology that is applicable to preventing Decarburization in Reheat applications. Fundamentals Control of the Decarburization of Steel has been standardized to the monitoring of - The Metal Temperature (Furnace Temperature) - The Atmosphere Concentration of Carbon Monoxide (CO) - The Atmosphere Concentration of Carbon Dioxide (CO2). Knowing the values of these three (3) parameters and the knowledge of - Saturated Austenite in Iron - The Alloy Content of the Steel - The Equilibrium Constant for the Controllable Chemical Reaction between the Steel and the Gas Atmosphere it can be determined if the Atmosphere will prevent Decarburization of the Steel.