Improved Processing Techniques for Inclusion-Free Steel for Bearing and Mechanical Component Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Crucible Industries
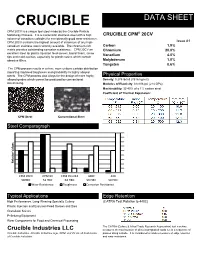
CRUCIBLE DATA SHEET CPM 20CV is a unique tool steel made by the Crucible Particle ® Metallurgy Process. It is a martensitic stainless steel with a high CRUCIBLE CPM 20CV volume of vanadium carbides for exceptionally good wear resistance. CPM 20CV contains the highest amount of chromium of any high- Issue #1 vanadium stainless steel currently available. The chromium rich Carbon 1.9% matrix provides outstanding corrosion resistance. CPM 20CV an Chromium 20.0% excellent steel for plastic injection feed screws, barrel liners, screw Vanadium 4.0% tips and mold cavities, especially for plastic resins which contain abrasive fillers. Molybdenum 1.0% Tungsten 0.6% The CPM process results in a finer, more uniform carbide distribution imparting improved toughness and grindability to highly alloyed steels. The CPM process also alloys for the design of more highly Physical Properties alloyed grades which cannot be produced by conventional Density: 0.275 lb/in3 (7616 kg/m3) steelmaking. Modulus of Elasticity: 31x106 psi (214 GPa) Machinability: 35-40% of a 1% carbon steel Coefficient of Thermal Expansion: CPM Steel Conventional Steel Steel Comparagraph Relative Relative Values CPM 20CV CPM 9V CPM Rex M4 440C 420 58HRC 53 HRC 63 HRC 56 HRC 50 HRC Wear Resistance Toughness Corrosion Resistance Typical Applications Edge Retention (CATRA Test Relative to 440C) High Performance, Long-Wearing Specialty Cutlery (CATRA Test Relative to 440C) Plastic Injection and Extrusion Feed Screws and Dies Granulator Knives Pelletizing Equipment Wear Components for Food and Chemical Processing The CATRA (Cutlery & Allied Trade Research Association) test machine Crucible Industries LLC measures the total number of silica impregnated cards cut in a sequence of Crucible Industries, Crucible Industries logo, CPM, and 9V are all trademarks passes along a blade. -

Table of Contents
Table of Contents INTRODUCTION…………………………………………………………………………………………………. 3 SUNY-ESF FIRST DESTINATION SURVEY: SUMMARY REPORT FOR 2016……………………... 7 Overall Responses…………………………………………………………………………………………. 7 Employment Information……………………..…………………………………………………………… 8 Continuing Education Information……………..…………………………………………………………. 11 Participation In University Programs…………..………………………………………………………….. 11 DEPARTMENT OF CHEMISTRY…………………..………………………………………………….……... 12 Overall Responses………………………………..…………………………………………………….….. 12 Employment Information………………………..………………………………………………………… 12 Continuing Education Information……………..…………………………………………………………. 14 Participation in University Programs……………..……………………………………………………….. 14 DEPARTMENT OF ENVIRONMENTAL and FOREST BIOLOGY……………………………..………. 15 Overall Responses…………………………………………………………………………………………. 15 Employment Information…………………………………………………………………….……………. 15 Continuing Education Information…………………………………………………………….………….. 21 Participation in University Programs……………………………………………………………………… 22 DEPARTMENT OF ENVIRONMENTAL RESOURCES ENGINEERING……………………..……….. 23 Overall Responses………………………………………………………………………………………….. 23 Employment Information…………………………………………………………………………………... 23 Continuing Education Information…………………………………………………………………………. 26 Participation in University Programs………………………………………………………………………. 27 DEPARTMENT OF ENVIRONMENTAL SCIENCE……………………………..………............................. 28 Overall Responses………………………………………………………………………………………….. 28 Employment Information………………………………………………………………………………….. 28 Continuing Education -

Annual Report of the Director Bureau of Standards to the Secretary Of
ANNUAL REPORT OF THE DIRECTOR BUREAU OF STANDARDS TO THE SECRETARY OF COMMERCE FOR THE FISCAL YEAR ENDED JUNE 30, 1915 WASHINGTON GOVERNMENT PRINTING OFFICE 1915 ANNUAL REPORT OF THE DIRECTOR BUREAU OF STANDARDS TO THE SECRETARY OF COMMERCE FOR THE FISCAL YEAR ENDED JUNE 30, 1915 WASHINGTON GOVERNMENT PRINTING OFFICE 1915 CONTENTS. Page. I. Functions, organization, and location 11 1. Standards of measurement 11 2. Pliysical constants 12 3. Standards of quality 12 4. Standards of performance 13 5. Standards of practice 14 6. Relation of the Bureau's work to the public 14 7. Relation of the Bureau's work to the Government service 16 8. Organization 17 9. Location ^ 18 II. Scientific and technical divisions 19 1. Weights and measures 19 Weights and balances 19 Capacity and length measures 19 Sieve testing 20 Volumetric glassware 20 Density tables for petroleum oils 20 Barometry 20 Testing of watches 21 Precision and empirical equations 22 Inspecting and testing scales 22 Operation of test-car equipment 23 Condition of track scales 23 Linear expansion of materials 24 Annual conference on weights and measures 25 Tolerances and specifications 25 So-called net-weight amendment to the pure-food law 26 Standard-barrel act 26 Information furnished on subjects pertaining to measures of length, mass, capacity, and time 26 Publications on weights and measures 27 2. Thermometry, pyrometry, and heat measurements 27 Platinum resistance thermometers 27 A AVheatstone bridge for resistance thermometry 28 A multiple junction thermoelectric thermometer 28 -

CPM 20CV Powder Metal Stainless Tool Steel
DATA SHEET Issue 1 ® CPM 20CV Powder Metal Stainless Tool Steel LATROBE SPECIALTY STEEL your source for all Crucible CPM products Typical Composition C Mn Si Cr W Mo V 1.90 0.30 0.30 20.00 0.60 1.00 4.00 CPM 20CV is a highly wear resistant, powder metallurgy stainless tool steel. The steel contains a large volume of extremely hard vanadium carbides, which provide excellent wear resistance. CPM 20CV contains the highest amount of chromium of any high-vanadium stainless steel currently available. The chromium-rich matrix provides outstanding corrosion resistance. CPM 20CV is a versatile stainless tool steel with a unique combination of high wear resistance, high corrosion resistance, good impact toughness, and excellent polishability. This combination of properties is the result of the fine grain size, small carbides, and superior cleanliness of the powder metallurgy (PM) microstructure. CPM 20CV excels in applications, which require a combination of high wear resistance and high corrosion resistance. The unique combination of properties makes CPM 20CV an excellent steel for plastic injection feed screws, barrel liners, screw tips, and mold cavities, especially for plastic resins which contain abrasive fillers. Other applications include food processing equipment, pelletizer knives, granulator knives, and high- performance custom knives. Relative Properties Toughness Wear Resistance Corrosion Resistance CPM 20CV CPM 9V CPM Rex M4 440C 420 58 HRC 53 HRC 63 HRC 56 HRC 50 HRC Physical Properties Edge Retention (CATRA Test Relative to 440C) -

CPM S60V Data Sheet
® DATA SHEET CPM S60V Typical Composition C Cr Mo V 2.15 17.0 .4 5.5 CPM S60V is a corrosion resistant, highly wear-resistant steel made by the Crucible Particle Metallurgy process. It is a martensitic stainless steel with a high volume of chromium and vanadium carbides for exceptionally good wear and corrosion resistance. S60V offers substantial improvements in wear resistance over 440C and D2, and other high chromium tool steels, with corrosion resistance much better than 440C. The CPM process results in a finer, more uniform carbide distribution imparting improved toughness and grind ability to high alloy steels. The CPM process also allows the design of more highly alloyed grades which cannot be produced by conventional steelmaking. Typical Applications: Plastic injection and extrusion feed screws, non-return valve components, pelletizing equipment, gate and nozzle inserts, industrial knives, slitters, and cutters, long-wearing specialty cutlery, injection molds and inserts, wear components for food and chemical processing, bearings, bushings, valves, rolls and gear pumps. Special Note to Knifemakers: The recommended hardness for blades is HRC 56/58, which is designed to provide an excellent combination of corrosion resistance, wear resistance and edge toughness. Mechanical Properties Hardness (1) Impact Toughness ft-lbs HRC Heat Treatment CPM S60V 56 (A) 16 CPM S60V 59 (B) 12 CPM S60V 60.5 (C) 11 1.) A=Hardened 1850°F (1010°C), double tempered 400°F (205°C). B=Hardened 1950°F (1065°C), double tempered 400°F (205°C). C=Hardened 2050°F (1120°C), double tempered 400°F (205°C). CONVENTIONAL STEEL CPM STEEL This data sheet is for informational purposes only. -
Sorted Alphabetically by Applicant Name Line
New York Power Authority ReCharge New York Applicants through June 25, 2020 - Sorted Alphabetically by Applicant Name Line Business Name Description of Applicant Street Address City State Zip Code NYS Assembly District(s) NY Senate District(s) Application Number 1 1886 Welcome Home Farm, Inc Community Food Pantry 6735 Route 9 Hudson NY 12534 103 41 8775 2 2 Twisted Farmers Farm (hops) 233 Clark Road Pulaski, NY 13142 Pulaski NY 13142 120 48 39930 3 24 Hour Tax & Accounting LLC Income tax preparation services N/A N/A NY N/A N/A N/A 18505 4 26 Cooper Ave., LLC Shop Painting 26 Cooper Avenue Tonawanda NY 14150 140 60 37389 5 2758 Trombley Road, LLC Bulk trucking services 2758 Trombley Road Weedsport NY 13166 130 50 96589 6 2758 Trombley Road, LLC Bulk trucking services 2758 Trombley Road Weedsport NY 13166 130 50 97098 7 346 Connecticut LLC Architect and developer 346 Connecticut Street Buffalo NY 14213 144 58 8794 8 365 Operating Company, LLC Data center & information technology services 500 Commack Road Commack NY 11725 8 2 87519 9 3M Company Cellulose sponges 305 Sawyer Ave. Tonawanda NY 14150 140 60 9379 10 425 Michigan Avenue LLC Medical/Educational office building 425 Michigan Avenue Buffalo NY 14203 141 63 23139 11 499 Syracuse City Centre Restoring and renovating buildings 499 S Warren St. Syracuse NY 13202 129 53 10117 12 5000 Group LLC Ownership/management of hotels, apartments & retail 5195 Main Street Williamsville NY 14221 148 61 11642 13 525 Wheat, LLC Start-up manufacturing facility 525 Wheatfield Street North Tonawanda NY 14120 140 62 11700 14 607 Phillips Street Acquisition, LLC (name to be determined for operating company) Frozen value added chicken products will be produced 607 Phillips Street Fulton NY 13069 120 48 14735 15 669 River Street LLC Incubator office space for new businesses 669 River Street Troy NY 12180 108 44 83448 16 850 East 138th Street, LLC Commercial laundry service 850 East 138th Street Bronx NY 10454 84 29 7773 17 A& Z Pharmaceutical Manufacturers pharmaceutical products 180 Oser Avenue Hauppauge NY 11788 8 2 40505 18 A. -

Standardized Parent Company Names for TRI Reporting
Standardized Parent Company Names for TRI Reporting This alphabetized list of TRI Reporting Year (RY) 2010 Parent Company names is provided here as a reference for facilities filing their RY 2011 reports using paper forms. For RY2011, the Agency is emphasizing the importance of accurate names for Parent Companies. Your facility may or may not have a Parent Company. Also, if you do have a Parent Company, please note that it is not necessarily listed here. Instructions Search for your standardized company name by pressing the CTRL+F keys. If your Parent Company is on this list, please write the name exactly as spelled and abbreviated here in Section 5.1 of the appropriate TRI Reporting Form. If your Parent Company is not on this list, please clearly write out the name of your parent company. In either case, please use ALL CAPITAL letters and DO NOT use periods. Please consult the most recent TRI Reporting Forms and Instructions (http://www.epa.gov/tri/report/index.htm) if you need additional information on reporting for reporting Parent Company names. Find your standardized company name on the alphabetical list below, or search for a name by pressing the CTRL+F keys Standardized Parent Company Names 3A COMPOSITES USA INC 3M CO 4-D CORROSION CONTROL SPECIALISTS INC 50% DAIRY FARMERS OF AMERICA 50% PRAIRIE FARM 88TH REGIONAL SUPPORT COMMAND A & A MANUFACTURING CO INC A & A READY MIX INC A & E INC A G SIMPSON AUTOMOTIVE INC A KEY 3 CASTING CO A MATRIX METALS CO LLC A O SMITH CORP A RAYMOND TINNERMAN MANUFACTURING INC A SCHULMAN INC A TEICHERT -
Crucible Data Sheet
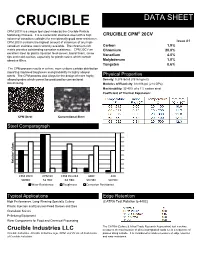
CRUCIBLE DATA SHEET CPM 20CV is a unique tool steel made by the Crucible Particle ® Metallurgy Process. It is a martensitic stainless steel with a high CRUCIBLE CPM 20CV volume of vanadium carbides for exceptionally good wear resistance. CPM 20CV contains the highest amount of chromium of any high- Issue #1 vanadium stainless steel currently available. The chromium rich Carbon 1.9% matrix provides outstanding corrosion resistance. CPM 20CV an Chromium 20.0% excellent steel for plastic injection feed screws, barrel liners, screw Vanadium 4.0% tips and mold cavities, especially for plastic resins which contain abrasive fillers. Molybdenum 1.0% Tungsten 0.6% The CPM process results in a finer, more uniform carbide distribution imparting improved toughness and grindability to highly alloyed steels. The CPM process also alloys for the design of more highly Physical Properties alloyed grades which cannot be produced by conventional Density: 0.275 lb/in3 (7616 kg/m3) steelmaking. Modulus of Elasticity: 31x106 psi (214 GPa) Machinability: 35-40% of a 1% carbon steel Coefficient of Thermal Expansion: CPM Steel Conventional Steel Steel Comparagraph Relative Relative Values CPM 20CV CPM 9V CPM Rex M4 440C 420 58HRC 53 HRC 63 HRC 56 HRC 50 HRC Wear Resistance Toughness Corrosion Resistance Typical Applications Edge Retention (CATRA Test Relative to 440C) High Performance, Long-Wearing Specialty Cutlery (CATRA Test Relative to 440C) Plastic Injection and Extrusion Feed Screws and Dies Granulator Knives Pelletizing Equipment Wear Components for Food and Chemical Processing The CATRA (Cutlery & Allied Trade Research Association) test machine Crucible Industries LLC measures the total number of silica impregnated cards cut in a sequence of Crucible Industries, Crucible Industries logo, CPM, and 9V are all trademarks passes along a blade. -

7313200007 DEC Permit Conditions FINAL Page 1 PERMIT Under The
Facility DEC ID: 7313200007 PERMIT Under the Environmental Conservation Law (ECL) IDENTIFICATION INFORMATION Permit Type: Air Title V Facility Permit ID: 7-3132-00007/00028 Effective Date: 06/07/2016 Expiration Date: 06/06/2021 Permit Issued To:CRUCIBLE INDUSTRIES LLC 575 STATE FAIR BLVD SYRACUSE, NY 13209 Contact: James Vreeland Crucible Industries LLC 575 State Fair Blvd Solvay, NY 13209 (315) 470-9234 Facility: CRUCIBLE INDUSTRIES 575 STATE FAIR BLVD GEDDES, NY 13209 Contact: James Vreeland Crucible Industries LLC 575 State Fair Blvd Solvay, NY 13209 (315) 470-9234 Description: Promulgation of 40 CFR 63, YYYYY requires all sources to obtain a Title V facility operating permit. Therefore, a Title V permit must be issued for this source; otherwise the source is a synthetic minor. By acceptance of this permit, the permittee agrees that the permit is contingent upon strict compliance with the ECL, all applicable regulations, the General Conditions specified and any Special Conditions included as part of this permit. Permit Administrator: ELIZABETH A TRACY 615 ERIE BLVD WEST SYRACUSE, NY 13204-2400 Authorized Signature: _________________________________ Date: ___ / ___ / _____ DEC Permit Conditions FINAL Page 1 Facility DEC ID: 7313200007 Notification of Other State Permittee Obligations Item A: Permittee Accepts Legal Responsibility and Agrees to Indemnification The permittee expressly agrees to indemnify and hold harmless the Department of Environmental Conservation of the State of New York, its representatives, employees and agents ("DEC") for all claims, suits, actions, and damages, to the extent attributable to the permittee's acts or omissions in connection with the compliance permittee's undertaking of activities in connection with, or operation and maintenance of, the facility or facilities authorized by the permit whether in compliance or not in any compliance with the terms and conditions of the permit. -

Standardized Parent Company Names for TRI Reporting
Standardized Parent Company Names for TRI Reporting This alphabetized list of TRI Reporting Year (RY) 2011 Parent Company names is provided here as a reference for facilities filing their RY 2012 reports using paper forms. For RY 2012, the Agency is emphasizing the importance of accurate names for Parent Companies. Your facility may or may not have a Parent Company. Also, if you do have a Parent Company, please note that it is not necessarily listed here. Instructions Search for your standardized company name by pressing the CTRL+F keys. If your Parent Company is on this list, please write the name exactly as spelled and abbreviated here in Section 5.1 of the appropriate TRI Reporting Form. If your Parent Company is not on this list, please clearly write out the name of your parent company. In either case, please use ALL CAPITAL letters and DO NOT use periods. Please consult the most recent TRI Reporting Forms and Instructions (http://www.epa.gov/tri/report/index.htm) if you need additional information on reporting for reporting Parent Company names. Find your standardized company name on the alphabetical list below, or search for a name by pressing the CTRL+F keys Standardized Parent Company Names 3A COMPOSITES USA INC 3F CHIMICA AMERICAS INC 3G MERMET CORP 3M CO 5N PLUS INC A & A MANUFACTURING CO INC A & A READY MIX INC A & E CUSTOM TRUCK A & E INC A FINKL & SONS CO A G SIMPSON AUTOMOTIVE INC A KEY 3 CASTING CO A MATRIX METALS CO LLC A O SMITH CORP A RAYMOND TINNERMAN MANUFACTURING INC A SCHULMAN INC A TEICHERT & SON INC A TO Z DRYING -

Stainless Steel
Stainless steel sion by blocking oxygen diffusion to the steel surface and blocks corrosion from spreading into the metal’s internal structure.[3] Passivation occurs only if the proportion of chromium is high enough and oxygen is present. 2 Properties Stainless steel cladding is used on the Walt Disney Concert Hall In metallurgy, stainless steel, also known as inox steel or inox from French inoxydable, is a steel alloy with a minimum of 10.5%[1] chromium content by mass. 1 Description Stainless steel (row 3) resists salt-water corrosion better than aluminum-bronze (row 1) or copper-nickel alloys (row 2) Stainless steel is used for corrosion-resistant tools such as this nutcracker Stainless steel does not readily corrode, rust or stain with water as ordinary steel does. However, it is not fully stain- proof in low-oxygen, high-salinity, or poor air-circulation environments.[2] There are different grades and surface finishes of stainless steel to suit the environment the al- loy must endure. Stainless steel is used where both the properties of steel and corrosion resistance are required. Stainless steel is not completely immune to corrosion in this desalination equipment Stainless steel differs from carbon steel by the amount of chromium present. Unprotected carbon steel rusts readily when exposed to air and moisture. This iron oxide film (the rust) is active and accelerates corrosion by forming 2.1 Oxidation more iron oxide; and, because of the greater volume of the iron oxide, this tends to flake and fall away. Stainless High oxidation resistance in air at ambient temperature steels contain sufficient chromium to form a passive film is normally achieved with addition of a minimum of of chromium oxide, which prevents further surface corro- 13% (by weight) chromium, and up to 26% is used for 1 2 2 PROPERTIES harsh environments.[4] The chromium forms a passivation 2.4 Organics layer of chromium(III) oxide (Cr2O3) when exposed to oxygen. -

Tooling Alloys
New since 1701 e TOOLING ALLOYS Zapp Materials Engineering GmbH The illustrations, drawings, dimensional and weight data and other information included in this brochure are intend ed only for the purposes of describing our products and represent nonbinding average values. They do not consti t ute quality data, nor can they be used as the basis for any guarantee of quality or durability. The applications present ed serve only as illustrations and can be construed neither as quality data nor as a guarantee in relation to the suit- abil ity of the material. This cannot substitute for compre hensive consultation on the selection of our products and on their use in a specific application. The brochure is not subject to change control. 0203 TOOLING ALLOYS CONTENT Zapp: from past generations – 04 for future generations 05 Tooling Alloys 08 ASP® steels 09 CPM® steels 10 Zapp special steels Advantages of powder metallurgical 11 high-performance steels CPM®, ASP® and zapp special steels 14 for cold-work applications 15 Tool steels 18 Services and special offers 24 Contact ZAPP: FROM PAST GENERATIONS – FOR FUTURE GENERATIONS 300 years of Zapp. In 1701 in Ründeroth, Germany, Hermann Zapp founded the company, which quickly became a specialist for high-grade, and high- performance steels. We deliver quickly and reliably thanks to a large net- work of selected sales partners and our own locations in Europe, Asia and the US. With our experience and expertise, we ensure that you can turn your ideas into reality. From past generations – for future generations! We are your partner for your projects and can carry out the first manu- facturing steps, such as cutting and straightening, prior to delivery.