Practical Bloomery Smelting W. H. Lee Sauder and Henry G. Williams III
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Register of Historic Places Multiple Property
NFS Form 10-900-b 0MB No. 1024-0018 (Jan. 1987) United States Department of the Interior National Park Service National Register of Historic Places Multipler Propertyr ' Documentation Form NATIONAL This form is for use in documenting multiple property groups relating to one or several historic contexts. See instructions in Guidelines for Completing National Register Forms (National Register Bulletin 16). Complete each item by marking "x" in the appropriate box or by entering the requested information. For additional space use continuation sheets (Form 10-900-a). Type all entries. A. Name of Multiple Property Listing ____Iron and Steel Resources of Pennsylvania, 1716-1945_______________ B. Associated Historic Contexts_____________________________ ~ ___Pennsylvania Iron and Steel Industry. 1716-1945_________________ C. Geographical Data Commonwealth of Pennsylvania continuation sheet D. Certification As the designated authority under the National Historic Preservation Act of 1966, as amended, J hereby certify that this documentation form meets the National Register documentation standards and sets forth requirements for the listing of related properties consistent with the National Register criteria. This submission meets the procedural and professional requiremerytS\set forth iri36JCFR PafrfsBOfcyid the Secretary of the Interior's Standards for Planning and Evaluation. Signature of certifying official Date / Brent D. Glass Pennsylvania Historical & Museum Commission State or Federal agency and bureau I, hereby, certify that this multiple -
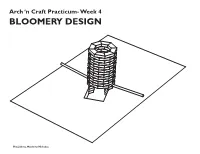
Bloomery Design
Arch ‘n Craft Practicum- Week 4 BLOOMERY DESIGN Hiu, Julieta, Matthew, Nicholas 7 3/4" The Stack: 3 3/4" In our ideal model we would use refractory bricks, either rectangular bricks or curved bricks which would help us make a circular 1'-8" stack. The stack would be around 3 feet tall with a 1 foot diameter for the inner wall of the stack. There will be openings about 3 brick lengths up(6-9 inches) from the ground on 1'-0" opposite ends of the stack. Attached to these openings will be our tuyeres which in turn will connect to our air sources. Building a circular stack would be beneficial to the type of air distribution we want to create within our furnace; using two angled tuyeres we would like air to move clockwise and counterclock- Bloomery Plan wise. We would like to line the inside of the stack with clay to provide further insulation. Refractory bricks would be an ideal material to use because the minerals they are com- posed of do not change chemically when 1'-0" exposed to high heat, some examples of these materials are aluminum, manganese, silica and zirconium. Other comments: 3'-0" Although we have opted to use refractory bricks, which would be practical for our intended purpose, we briefly considered using Vycor glass which can withstand high tem- peratures of up to 900 degrees celsius or approximately 1652 degrees Fahrenheit, which Tuyere height: Pitched -10from horizontal 7" would be enough for our purposes. Bloomery Section Ore/ Charcoal: Our group has decided to use Magnetite ore along with charcoal for the smelt. -

On a Mathematical Model for Case Hardening of Steel
On a mathematical model for case hardening of steel vorgelegt von Dott.ssa Lucia Panizzi Von der Fakultät II ‚ Mathematik und Naturwissenschaften der Technischen Universität Berlin zur Erlangung des akademischen Grades Doktor der Naturwissenschaften Dr. rer. nat. sowie der Classe di Scienze der Scuola Normale Superiore di Pisa als Diploma di Perfezionamento in Matematica per la Tecnologia e l’Industria genehmigte Dissertation Promotionsausschuss: Berichter/Gutachter: Prof. A. Fasano (Univerisità di Firenze) Berichter/Gutachter: Prof. D. Hömberg (Technische Universität Berlin) Gutachter: Prof. L. Formaggia (Politecnico di Milano) Gutachter: Prof. M. Primicerio (Università di Firenze) Gutachter: Prof. F. Tröltzsch (Technische Universität Berlin) Gutachter: Prof. P. Wittbold (Technische Universität Berlin) Tag der wissenschaftlichen Aussprache: 05.03.2010 Berlin 2010 D83 i Eidesstattliche Versicherung Hiermit erkläre ich, dass die vorliegende Dissertation: On a mathematical model for case hardening of steel selbständig verfasst wurde. Die benutzten Hilfsmittel und Quellen wurden von mir angegeben, weitere wurden nicht verwendet. ii Erklärung Hiermit erkläre ich, dass die Anmeldung meiner Promotionsabsicht früher nicht bei einer anderen Hochschule oder einer anderen Fakultät beantragt wurde. Teile meiner Dissertation sind darüber hinaus schon veröffentlich worden, welche im Folgenden aufgelistet sind: • D. Hömberg, A. Fasano, L. Panizzi A mathematical model for case hardening of steel. angenommen zur Veröffentlichung in "Mathematical Models and Methods in Applied Sciences" (M3AS), 2009. • P. Krejčí, L. Panizzi. Regularity and uniqueness in quasilinear parabolic systems. angenommen zur Veröffentlichung in "Applications of Mathematics", 2009. iii Compendio Nonostante la disponibilità di numerosi nuovi materiali, l’acciaio rimane il ma- teriale di base della moderna società industriale. L’uso dell’ acciaio con peculiari caratteristiche (durezza, resistenza all’uso, malleabilità etc.) è perciò assai diffuso in molti settori della tecnica. -

Hopewell Village
IRONMAKING IN EARLY AMERICA the Revolutionary armies, is representative of (such as Valley Forge) to be made into the tougher the hundreds of ironmaking communities that and less brittle wrought iron. This was used to Hopewell In the early days of colonial America, iron tools supplied the iron needs of the growing nation. make tools, hardware, and horseshoes. and household items were brought over from Europe by the settlers or imported at a high cost. Until surpassed by more modern methods, cold- HOPEWELL FURNACE Village The colonists, early recognizing the need to manu blast charcoal-burning furnaces, such as that NATIONAL HISTORIC SITE • PENNSYLVANIA facture their own iron, set up a number of iron at Hopewell, supplied all the iron. These furnaces In an age when most businesses were operated by works, notably at Falling Creek, Va., and Saugus, consumed about 1 acre of trees a day for fuel, one or two men in a shop, Hopewell employed at Mass. Operations gradually spread throughout so they had to be located in rural areas close to least 65 men, with some responsible for two or the colonies, and by the end of the 1700's, south a timber supply. more jobs. As the nearest town was many miles eastern Pennsylvania had become the industry's away, the ironmaster built a store to supply his center. Hopewell Village, founded by Mark Bird Since the pig iron produced by these furnaces workers, many of whom lived in company-owned in 1770 in time to supply cannon and shot for had a limited use, much of it was sent to forges homes. -

UNDERSTANDING and MEASURING DECARBURIZATION Understanding the Forces Behind Decarburization Is the First Step Toward Minimizing Its Detrimental Effects
22 UNDERSTANDING AND MEASURING DECARBURIZATION Understanding the forces behind decarburization is the first step toward minimizing its detrimental effects. George F. Vander Voort, FASM*, Struers Inc. (Consultant), Cleveland ecarburization is detrimental to crostructure of carbon contents close to the maximum depth of decarburization the wear life and fatigue life of the core may be difficult to discern. The is established. Because the carbon dif- steel heat-treated components. MAD determined by hardness traverse fusion rate increases with temperature ADVANCED MATERIALS & PROCESSES | FEBRUARY 2015 2015 FEBRUARY | & PROCESSES MATERIALS ADVANCED D This article explores some factors that may be slightly shallower than that de- when the structure is fully austenitic, cause decarburization while concentrat- termined by quantitative carbon analysis MAD also increases as temperature rises ing on its measurement. In most produc- with the electron microprobe. This is es- above the Ac3. For temperatures in the tion tests, light microscopes are used to pecially true when the bulk carbon con- two-phase region, between the Ac1 and scan the surface of a polished and etched tent exceeds about 0.45 wt%, as the rela- Ac3, the process is more complex. Carbon cross-section to find what appears to be tionship between carbon in the austenite diffusion rates in ferrite and austenite the greatest depth of total carbon loss before quenching to form martensite and are different, and are influenced by both (free-ferrite depth, or FFD) and the great- the as-quenched hardness loses its linear temperature and composition. est depth of combined FFD and partial nature above this carbon level. Decarburization is a serious prob- loss of carbon to determine the maxi- lem because surface properties are infe- mum affected depth (MAD). -

An Introduction to Nitriding
01_Nitriding.qxd 9/30/03 9:58 AM Page 1 © 2003 ASM International. All Rights Reserved. www.asminternational.org Practical Nitriding and Ferritic Nitrocarburizing (#06950G) CHAPTER 1 An Introduction to Nitriding THE NITRIDING PROCESS, first developed in the early 1900s, con- tinues to play an important role in many industrial applications. Along with the derivative nitrocarburizing process, nitriding often is used in the manufacture of aircraft, bearings, automotive components, textile machin- ery, and turbine generation systems. Though wrapped in a bit of “alchemi- cal mystery,” it remains the simplest of the case hardening techniques. The secret of the nitriding process is that it does not require a phase change from ferrite to austenite, nor does it require a further change from austenite to martensite. In other words, the steel remains in the ferrite phase (or cementite, depending on alloy composition) during the complete proce- dure. This means that the molecular structure of the ferrite (body-centered cubic, or bcc, lattice) does not change its configuration or grow into the face-centered cubic (fcc) lattice characteristic of austenite, as occurs in more conventional methods such as carburizing. Furthermore, because only free cooling takes place, rather than rapid cooling or quenching, no subsequent transformation from austenite to martensite occurs. Again, there is no molecular size change and, more importantly, no dimensional change, only slight growth due to the volumetric change of the steel sur- face caused by the nitrogen diffusion. What can (and does) produce distor- tion are the induced surface stresses being released by the heat of the process, causing movement in the form of twisting and bending. -

20. Iron and Steel Part II Copy
USES FOR TYPES OF STEELS • Low carbon steel (0.08 - 0.35 % carbon) is ductile with low brittleness. It is is used for ANCIENT IRON auto body parts, home appliances, tin cans, I beams for construction. AND STEEL • Medium carbon steel (0.35 - 0.5 % carbon) (Part II) is used as crankshaft, gears, railroad axels. (Part II) They are difficult to weld. • High carbon steel (> 0.5 % carbon) is used for railroad wheels and rails, wrenches, steel cable, tools, dies, piano wire etc. BLAST FURNACE CAST IRON (PIG IRON) • Contains 1.5 - 5 % carbon. • Its melting point is 1130 oC. • The metal will shatter with a hard blow. • Carbon in the form of graphite exists as flakes • Graphite serves as a lubricant (excellent bearing material). 1 Cast Grey Iron Blast furnace at Cast Grey Iron Karabük Iron works Cast Gray Iron Graphite flakes THE FINERY CAST IRON OBJECTS THE FINERY Cast cannon 15th. Cent. 2 INDIRECT METHOD OF STEEL BESSEMER PROCESS PRODUCTION (THE BESSEMER PROCESS) Crude cast iron from the blast furnace is remelted in a hearth (the finary) and subjected to a forced draught of air. The excess carbon is oxidized to carbon dioxide. The refined pig iron which is called malleable iron is now ready for final forging. BESSEMER PROCESS FORMS OF IRON CAST IRON Decarburization From Blast Furnace M.P. 1130oC 1,5-4.5 % C Bessemer STEEL Process M.P. 1400o C 0.1-0.9 % C WROUGHT IRON From Bloomery Cementation M.P 1535o C 0.06 % C Carburization 3 DIFFERENCES BETWEEN BLOOMERY ALLOY STEELS AND BLAST FURNACE • Increase in temperature (1300 - 1400oC) • Alloy steel describes steel that contain one or more alloying elements in addition to carbon. -

AISI | Electric Arc Furnace Steelmaking
http://www.steel.org/AM/Template.cfm?Section=Articles3&TEMPLATE=/CM/HTMLDisplay.cfm&CONTENTID=12308 Home Steelworks Home Electric Arc Furnace Steelmaking By Jeremy A. T. Jones, Nupro Corporation SIGN UP to receive AISI's FREE e-news! Read the latest. Email: Name: Join Courtesy of Mannesmann Demag Corp. FURNACE OPERATIONS The electric arc furnace operates as a batch melting process producing batches of molten steel known "heats". The electric arc furnace operating cycle is called the tap-to-tap cycle and is made up of the following operations: Furnace charging Melting Refining De-slagging Tapping Furnace turn-around Modern operations aim for a tap-to-tap time of less than 60 minutes. Some twin shell furnace operations are achieving tap-to-tap times of 35 to 40 minutes. 10/3/2008 9:36 AM http://www.steel.org/AM/Template.cfm?Section=Articles3&TEMPLATE=/CM/HTMLDisplay.cfm&CONTENTID=12308 Furnace Charging The first step in the production of any heat is to select the grade of steel to be made. Usually a schedule is developed prior to each production shift. Thus the melter will know in advance the schedule for his shift. The scrap yard operator will prepare buckets of scrap according to the needs of the melter. Preparation of the charge bucket is an important operation, not only to ensure proper melt-in chemistry but also to ensure good melting conditions. The scrap must be layered in the bucket according to size and density to promote the rapid formation of a liquid pool of steel in the hearth while providing protection for the sidewalls and roof from electric arc radiation. -

Blast Furnace Stove Dome and Hot Blast Main
BLAST FURNACE STOVE DOME AND HOT BLAST MAIN The quality and composition of iron produced in the blast furnace is directly related to the hearth temperature. This, in turn, is dependent on the temperature of the hot blast delivered from the blast furnace stoves. To maximise the efficiency of the stoves, they are operated at high temperatures, close to the safe working limit of the refractories. This makes it critical to carefully monitor the stove temperature. BLAST FURNACES AND STOVES Blast furnaces heat iron ore to a period of accumulation, flow is produce the iron required as a reversed, and the hot stove is used raw material for steel-making. For to preheat the incoming air. efficient operation, the air is heated Stoves are alternated, storing heat before being sent into the furnace. and dissipating heat on a regular This ‘hot blast’ technique – flow reversal plan. Many blast preheating air blown into the furnaces are serviced by three or blast furnace – dates back to the more stoves, so that while two are Industrial Revolution, and was being heated, the air blast can pass developed to permit higher through the regenerative chamber furnace temperatures, increasing of the third stove on its way to the the furnace capacity. furnace. Preheating the air intensifies and Accurate monitoring of the stove accelerates the burning of the temperature supports efficient coke. A blast furnace fed with operation – higher temperatures air preheated to between 900- are more efficient and, by reducing 1250oC (1652-2282oF) can generate coke consumption, are more cost- smelting temperatures of about effective. -

CONTROL of DECARBURIZATION of STEEL Paul Shefsiek
CONTROL OF DECARBURIZATION OF STEEL Paul Shefsiek Introduction Historically, heating steel for forming, forging or rolling, was done in electrical resistance or natural gas heated furnaces. It was inevitable that these furnaces contained Oxygen and Decarburization of the steel surface occurred. This decarburization was either ignored or minimized by coating the steel with “ stopoff “. Also, this decarburization was minimized through the use nitrogen atmosphere furnaces. But, decarburization could not be reduced to acceptable limits until the Chemical Potential of the Carbon in the furnace atmosphere matched the dissolved Carbon in the steel. The Furnace Industry that provides Equipment for the processing of steel, because of the temperature ranges involved, divided itself into two categories, namely, Reheat and Heat Treating. Each has its own special Technology. But, because of this division, quite often one division does not know the Technology of the other. In some cases this has been unfortunate. If the Reheat side of the Industry had the Carburizing Technology of the Heat Treating side, this Technology could have been applied to applications where preventing Decarburizing was a requirement. Therefore, the purpose of the following discussion is to separate out that part of Carburizing Technology that is applicable to preventing Decarburization in Reheat applications. Fundamentals Control of the Decarburization of Steel has been standardized to the monitoring of - The Metal Temperature (Furnace Temperature) - The Atmosphere Concentration of Carbon Monoxide (CO) - The Atmosphere Concentration of Carbon Dioxide (CO2). Knowing the values of these three (3) parameters and the knowledge of - Saturated Austenite in Iron - The Alloy Content of the Steel - The Equilibrium Constant for the Controllable Chemical Reaction between the Steel and the Gas Atmosphere it can be determined if the Atmosphere will prevent Decarburization of the Steel. -

1.1%Mn--0.05%C Steel Sheets by Silicon Dioxide and Development Of
Materials Transactions, Vol. 44, No. 6 (2003) pp. 1096 to 1105 #2003 The Japan Institute of Metals Decarburization of 3%Si–1.1%Mn–0.05%C Steel Sheets by Silicon Dioxide and Development of {100}h012i Texture* Toshiro Tomida Corporate Research & Development Laboratories, Sumitomo Metal Industries, Ltd., Amagasaki 660-0891, Japan Decarburization of silicon steel sheets by annealing with oxide separators has been found to cause a high degree of {100}-texture development. Cold-rolled Fe–3%Si–1.1%Mn–0.05%C sheets of 0.35 mm thickness were laminated with separators containing SiO2. They were then annealed under a reduced pressure at about 1300 K in a ferrite and austenite two phase region. It has been observed that carbon concentration notably decreases down to 0.001% during the lamination annealing. Thus an almost complete decarburization of sheet steels was possible, whereas no oxidation of silicon as well as manganese and iron occurred. Associated with decarburization, columnar ferrite grains grew inward from sheet surfaces due to the phase transformation from austenite to ferrite. A {100}h012i texture dramatically developed in the columnar grains. Fully decarburized materials consisted of grains of 0.6 mm diameter, more than 90% of which were closely aligned with {100}h012i orientation. Another aspect of great interest in the grain structure after decarburization was that there existed convoluted domains of a few mm in width, in which dozens of grains were oriented in a single variant of the texture, (100)[012] or (100)[021]. The decarburization is considered to be caused by the thermo-chemical reaction, 2C+SiO2!Si+2CO. -

3. Classification 4. Owner of Property 5. Location of Legal Description 6
NATIONAL HISTORIC LANDMARK NPS Form 10-900 OMB No. 1024-0018 (3-82) Exp. 10-31-84 United States Department of the interior National Park Semes For NPS use only Of received date entered See instructions in How to Complete National Register Forms Type all entries—complete applicable sections historic CORNWALL IRON FURNACE NATIONAL HISTORIC LANDMARK DISTRICT and or common In irregular pattern along Rexmont Road and Boyd street & number street_________ _ _______ __________________—— no* city, town Cornwall Borough ——vicinity of state Pennsylvania code 42 county Lebanon code 075 3. Classification Category Ownership Status Present Use X district __ public X occupied agriculture _X_ museum building(s) private X unoccupied commercial park structure both work in progress educational _X_ private residence site Public Acquisition Accessible entertainment religious object in process yes: restricted government efiantifir being considered _X- yes: unrestricted X industrial transportation no military ...X other: 4. Owner of Property name Multiple - see continuation sheet street & number city, town __ vicinity of state 5. Location of Legal Description courthouse, registry of deeds, etc. T.P hanon County Courthouse street & number South 8th Street city, town Lebanon state PA 6. Representation in Existing Surveys title Historic Sites Survey has this property been determined eligible? yes no date 1966 _ federal county local depository for survey records National Park Service city, town Washington state D.C. 7. Description Condition Check one Check one X excellent deteriorated unaltered XL original site good ruins _X_ altered moved date fair unexposed Describe the present and original (if known) physical appearance The Cornwall Iron Furnace National Historic Landmark contains three interrelated major elements.