Metallography and Microstructure of Ancient and Historic Metals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Manufacturing Processes
Module 1 Classification of Metal Removal Processes and Machine tools Version 2 ME IIT, Kharagpur Lesson 2 Basic working principle, configuration, specification and classification of machine tools Version 2 ME IIT, Kharagpur Instructional Objectives At the end of this lesson, the students should be able to : (a) Describe the basic functional principles of machine tools (i) Illustrate the concept of Generatrix and Directrix (ii) Demonstrate Tool – work motions (iii) Give idea about machine tool drives (b) Show configuration of basic machine tools and state their uses (c) Give examples of machine tools - specification (d) Classify machine tools broadly. Basic functional principles of machine tool operations Machine Tools produce desired geometrical surfaces on solid bodies (preformed blanks) and for that they are basically comprised of; • Devices for firmly holding the tool and work • Drives for providing power and motions to the tool and work • Kinematic system to transmit motion and power from the sources to the tool-work • Automation and control systems • Structural body to support and accommodate those systems with sufficient strength and rigidity. For material removal by machining, the work and the tool need relative movements and those motions and required power are derived from the power source(s) and transmitted through the kinematic system(s) comprised of a number and type of mechanisms. (i) Concept of Generatrix and Directrix • Generation of flat surface The principle is shown in Fig. 2.1 where on a flat plain a straight line called Generatrix (G) is traversed in a perpendicular direction called Directrix (D) resulting a flat surface. • Generation of cylindrical surfaces The principles of production of various cylindrical surfaces (of revolution) are shown in Fig. -

Treatise on Combined Metalworking Techniques: Forged Elements and Chased Raised Shapes Bonnie Gallagher
Rochester Institute of Technology RIT Scholar Works Theses Thesis/Dissertation Collections 1972 Treatise on combined metalworking techniques: forged elements and chased raised shapes Bonnie Gallagher Follow this and additional works at: http://scholarworks.rit.edu/theses Recommended Citation Gallagher, Bonnie, "Treatise on combined metalworking techniques: forged elements and chased raised shapes" (1972). Thesis. Rochester Institute of Technology. Accessed from This Thesis is brought to you for free and open access by the Thesis/Dissertation Collections at RIT Scholar Works. It has been accepted for inclusion in Theses by an authorized administrator of RIT Scholar Works. For more information, please contact [email protected]. TREATISE ON COMBINED METALWORKING TECHNIQUES i FORGED ELEMENTS AND CHASED RAISED SHAPES TREATISE ON. COMBINED METALWORKING TECHNIQUES t FORGED ELEMENTS AND CHASED RAISED SHAPES BONNIE JEANNE GALLAGHER CANDIDATE FOR THE MASTER OF FINE ARTS IN THE COLLEGE OF FINE AND APPLIED ARTS OF THE ROCHESTER INSTITUTE OF TECHNOLOGY AUGUST ( 1972 ADVISOR: HANS CHRISTENSEN t " ^ <bV DEDICATION FORM MUST GIVE FORTH THE SPIRIT FORM IS THE MANNER IN WHICH THE SPIRIT IS EXPRESSED ELIEL SAARINAN IN MEMORY OF MY FATHER, WHO LONGED FOR HIS CHILDREN TO HAVE THE OPPORTUNITY TO HAVE THE EDUCATION HE NEVER HAD THE FORTUNE TO OBTAIN. vi PREFACE Although the processes of raising, forging, and chasing of metal have been covered in most technical books, to date there is no major source which deals with the functional and aesthetic requirements -

FIG. 7001 Flexible Coupling
COUPLINGS FIG. 7001 Flexible Coupling The Gruvlok® Fig. 7001 Coupling forms a flexible grooved end pipe joint connection with the versatility for a wide range of applications. Services include mechanical and plumbing, process piping, mining and oil field piping, and many others. The coupling design supplies optimum strength for working pressures to 1000 PSl (69 bar) without excessive casting weight. The flexible design eases pipe and equipment installation while providing the designed-in benefit of reducing pipeline noise and vibration transmission without the addition of special components. To ease coupling handling and assembly and to assure consistent quality, sizes 1" through 14" couplings have two 180° segment housings, 16" have three 120˚ segment housings, and 18" through 24" sizes have four 90° segment housings, while the 28" O.D. and 30" O.D. For Listings/Approval Details and Limitations, visit our website at www.anvilintl.com or couplings have six 60° segment housings. The 28" O.D. and 30" O.D. are weld-ring couplings. contact an Anvil® Sales Representative. MATERIAL SPECIFICATIONS BOLTS: q Grade “T” Nitrile (Orange color code) SAE J429, Grade 5, Zinc Electroplated -20°F to 180°F (Service Temperature Range)(-29°C to 82°C) ISO 898-1, Class 8.8, Zinc Electroplated followed by a Yellow Chromate Dip Recommended for petroleum applications. Air with oil vapors and HEAVY HEX NUTS: vegetable and mineral oils. ASTM A563, Grade A, Zinc Electroplated NOT FOR USE IN HOT WATER OR HOT AIR ISO 898-2, Class 8.8, Zinc Electroplated followed by a Yellow Chromate Dip q Grade “O” Fluoro-Elastomer (Blue color code) HARDWARE KITS: Size Range: 1" - 12" (C style only) 3 20°F to 300°F (Service Temperature Range)(-29°C to 149°C) q 304 Stainless Steel (available in sizes up to /4") Kit includes: (2) Bolts per ASTM A193, Grade B8 and Recommended for high temperature resistance to oxidizing acids, (2) Heavy Hex Nuts per ASTM A194, Grade 8. -

Materials & Process
Sculpture: Materials & Process Teaching Resource Developed by Molly Kysar 2001 Flora Street Dallas, TX 75201 Tel 214.242.5100 Fax 214.242.5155 NasherSculptureCenter.org INDEX INTRODUCTION 3 WORKS OF ART 4 BRONZE Material & Process 5-8 Auguste Rodin, Eve, 1881 9-10 George Segal, Rush Hour, 1983 11-13 PLASTER Material & Process 14-16 Henri Matisse, Madeleine I, 1901 17-18 Pablo Picasso, Head of a Woman (Fernande), 1909 19-20 STEEL Material & Process 21-22 Antony Gormley, Quantum Cloud XX (tornado), 2000 23-24 Mark di Suvero, Eviva Amore, 2001 24-25 GLOSSARY 26 RESOURCES 27 ALL IMAGES OF WORKS OF ART ARE PROTECTED UNDER COPYRIGHT. ANY USES OTHER THAN FOR EDUCATIONAL PURPOSES ARE STRICTLY FORBIDDEN. 2 Introduction This resource is designed to introduce students in 4th-12th grades to the materials and processes used in modern and traditional sculpture, specifically bronze, plaster, and steel. The featured sculptures, drawn from the collection of the Nasher Sculpture Center, range from 1881 to 2001 and represent only some of the many materials and processes used by artists whose works of art are in the collection. Images from this packet are also available in a PowerPoint presentation for use in the classroom, available at nashersculpturecenter.org. DISCUSS WITH YOUR STUDENTS Artists can use almost any material to create a work of art. When an artist is deciding which material to use, he or she may consider how that particular material will help express his or her ideas. Where have students seen bronze before? Olympic medals, statues… Plaster? Casts for broken bones, texture or decoration on walls.. -

Vibrations in Metal Cutting Measurement, Analysis and Reduction
Vibrations in Metal Cutting Measurement, Analysis and Reduction Linus Pettersson Ronneby, March 2002 Department of Telecommunications and Signal Processing Blekinge Institute of Technology 372 25 Ronneby, Sweden c Linus Pettersson Licentiate Dissertation Series No. 01/02 ISSN 1650-2140 ISBN 91-7295-008-0 Published 2002 Printed by Kaserntryckeriet AB Karlskrona 2002 Sweden v Abstract Vibration and noise in metal cutting are ubiquitous problems in the workshop. The turning operation is one kind of metal cutting that exhibits vibration related problems. Today the industry aims at smaller tolerances in surface finish. Harder regulations in terms of the noise levels in the operator environment are also central. One step towards a solution to the noise and vibration problems is to investigate what kind of vibrations that are present in a turning operation. The vibrations in a boring operation have been put under scrutiny in the first part of this thesis. Analytical models have been compared with experimental results and the vibration pattern has been determined. The second part of the thesis deals with active vibration control in external turning operations. By embedding a piezo-ceramic actuator and an accelerometer into a tool holder it was possible to obtain a solution that can be fitted in a standard lathe. The control system consists of the active tool holder, a control system based on the filtered-X LMS algorithm and an amplifier designed for capacitive loads. The vibration level using this technique can be reduced by as much as 40 dB during an external turning operation. vii Preface The work presented in this licentiate thesis has been performed at the department of Telecommunications and Signal Processing at Blekinge Institute of Technology. -

Structural Treatment of a Monumental Japanese Bronze Eagle from the Meiji Period Author(S): Marianne Russell-Marti and Robert F
Article: Structural treatment of a monumental Japanese bronze eagle from the Meiji period Author(s): Marianne Russell-Marti and Robert F. Marti Source: Objects Specialty Group Postprints, Volume Three, 1995 Pages: 39-63 Compilers: Julie Lauffenburger and Virginia Greene th © 1996 by The American Institute for Conservation of Historic & Artistic Works, 1156 15 Street NW, Suite 320, Washington, DC 20005. (202) 452-9545 www.conservation-us.org Under a licensing agreement, individual authors retain copyright to their work and extend publications rights to the American Institute for Conservation. Objects Specialty Group Postprints is published annually by the Objects Specialty Group (OSG) of the American Institute for Conservation of Historic & Artistic Works (AIC). A membership benefit of the Objects Specialty Group, Objects Specialty Group Postprints is mainly comprised of papers presented at OSG sessions at AIC Annual Meetings and is intended to inform and educate conservation-related disciplines. Papers presented in Objects Specialty Group Postprints, Volume Three, 1995 have been edited for clarity and content but have not undergone a formal process of peer review. This publication is primarily intended for the members of the Objects Specialty Group of the American Institute for Conservation of Historic & Artistic Works. Responsibility for the methods and materials described herein rests solely with the authors, whose articles should not be considered official statements of the OSG or the AIC. The OSG is an approved division of the AIC but does not necessarily represent the AIC policy or opinions. STRUCTURAL TREATMENT OF A MONUMENTAL JAPANESE BRONZE EAGLE FROM THE MEIJI PERIOD Marianne Russell-Marti and Robert F. -

70/30 Cupronickel
70/30 cupronickel www.columbiametals.com CN107 / C71500 / CW354H / DEF STAN 02-780 [email protected] 70/30 is a copper nickel alloy noted for its excellent resistance to corrosion, erosion and pitting combined with a good strength, workability and weldability. It has enjoyed a long and successful history in the marine sector that has since extended to industries including offshore oil and gas, power generation, desalination and cooling plants. 70/30 cupronickel The most popular specifications covering this alloy are the CN107 and C71500 designations, although the Naval Engineering specifications DEF STAN 02-780 or NES 780 offer tighter controls on impurities and mechanical properties together with a mandatory impact value. 70/30 is best renowned for its excellent corrosion and erosion resistance, especially in marine environments. Its corrosion resistance is improved in higher velocity waters (up to 4.5m/s) and polluted seawater. It is also highly resistant to stress corrosion cracking and corrosion fatigue and has a high retention of mechanical properties from sub-zero temperatures to ~300oC. In the annealed condition, 70/30 offers moderate strength levels which enable it to be used in more demanding applications. In addition, it displays inherent resistance to biofouling. A protective oxide film forms naturally over the material during the initial period of use, creating an inhospitable surface that deters marine growth. 70/30 responds well to most fabrication processes and is readily hot and cold worked. It is also easily joined by soldering, brazing and a variety of welding methods. With good hot and cold formability and a malleability approaching that of copper, 70/30 does not harden rapidly, lending the material to drawing and spinning. -

An Analysis of the Metal Finds from the Ninth-Century Metalworking
Western Michigan University ScholarWorks at WMU Master's Theses Graduate College 8-2017 An Analysis of the Metal Finds from the Ninth-Century Metalworking Site at Bamburgh Castle in the Context of Ferrous and Non-Ferrous Metalworking in Middle- and Late-Saxon England Julie Polcrack Follow this and additional works at: https://scholarworks.wmich.edu/masters_theses Part of the Medieval History Commons Recommended Citation Polcrack, Julie, "An Analysis of the Metal Finds from the Ninth-Century Metalworking Site at Bamburgh Castle in the Context of Ferrous and Non-Ferrous Metalworking in Middle- and Late-Saxon England" (2017). Master's Theses. 1510. https://scholarworks.wmich.edu/masters_theses/1510 This Masters Thesis-Open Access is brought to you for free and open access by the Graduate College at ScholarWorks at WMU. It has been accepted for inclusion in Master's Theses by an authorized administrator of ScholarWorks at WMU. For more information, please contact [email protected]. AN ANALYSIS OF THE METAL FINDS FROM THE NINTH-CENTURY METALWORKING SITE AT BAMBURGH CASTLE IN THE CONTEXT OF FERROUS AND NON-FERROUS METALWORKING IN MIDDLE- AND LATE-SAXON ENGLAND by Julie Polcrack A thesis submitted to the Graduate College in partial fulfillment of the requirements for the degree of Master of Arts The Medieval Institute Western Michigan University August 2017 Thesis Committee: Jana Schulman, Ph.D., Chair Robert Berkhofer, Ph.D. Graeme Young, B.Sc. AN ANALYSIS OF THE METAL FINDS FROM THE NINTH-CENTURY METALWORKING SITE AT BAMBURGH CASTLE IN THE CONTEXT OF FERROUS AND NON-FERROUS METALWORKING IN MIDDLE- AND LATE-SAXON ENGLAND Julie Polcrack, M.A. -

Colonial American Jobs
Name: ____________________________ Colonial American Jobs Match each colonial occupation with its description. If you're not sure of the answers, use a computer or dictionary to look up the words. 1. _____ blacksmith a. ground corn and wheat to make flour 2. _____ cobbler b. made and repaired clothing, such as suits and dresses, from fabric 3. _____ cooper c. made clothing and blankets from animal hides; made saddles for horses 4. _____ wheelwright d. printed newspapers and signs with a printing press 5. _____ silversmith e. made horseshoes and farm equipment from iron and steel 6. _____ miller f. made and repaired wagons and wheels 7. _____ milliner g. made and sold hats 8. _____ tanner h. repaired, altered, and made firearms 9. _____ apothecary i. made and fixed shoes 10. _____ tailor j. made dishes, spoons, and cups from pewter (silver) 11. _____ gunsmith k. made barrels out of wood 12. _____ printer l. mixed herbs to make medicine for the sick Super Teacher Worksheets - www.superteacherworksheets.com ANSWER KEY Colonial American Jobs Match each colonial occupation with its description. If you're not sure of the answers, use a computer or dictionary to look up the words. 1. e blacksmith a. ground corn and wheat to make flour 2. i cobbler b. made and repaired clothing, such as suits and dresses, from fabric 3. k cooper c. made clothing and blankets from animal hides; made saddles for horses 4. f wheelwright d. printed newspapers and signs with a printing press 5. j silversmith e. made horseshoes and farm equipment from iron and steel 6. -
Bloomery Design
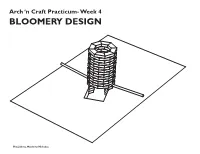
Arch ‘n Craft Practicum- Week 4 BLOOMERY DESIGN Hiu, Julieta, Matthew, Nicholas 7 3/4" The Stack: 3 3/4" In our ideal model we would use refractory bricks, either rectangular bricks or curved bricks which would help us make a circular 1'-8" stack. The stack would be around 3 feet tall with a 1 foot diameter for the inner wall of the stack. There will be openings about 3 brick lengths up(6-9 inches) from the ground on 1'-0" opposite ends of the stack. Attached to these openings will be our tuyeres which in turn will connect to our air sources. Building a circular stack would be beneficial to the type of air distribution we want to create within our furnace; using two angled tuyeres we would like air to move clockwise and counterclock- Bloomery Plan wise. We would like to line the inside of the stack with clay to provide further insulation. Refractory bricks would be an ideal material to use because the minerals they are com- posed of do not change chemically when 1'-0" exposed to high heat, some examples of these materials are aluminum, manganese, silica and zirconium. Other comments: 3'-0" Although we have opted to use refractory bricks, which would be practical for our intended purpose, we briefly considered using Vycor glass which can withstand high tem- peratures of up to 900 degrees celsius or approximately 1652 degrees Fahrenheit, which Tuyere height: Pitched -10from horizontal 7" would be enough for our purposes. Bloomery Section Ore/ Charcoal: Our group has decided to use Magnetite ore along with charcoal for the smelt. -

Tumbaga-Metal Figures from Panama: a Conservation Initiative to Save a Fragile Coll
SPRING 2006 IssuE A PUBLICATION OF THE PEABODY MUSEUM AND THE DEPARTMENT OF ANTHROPOLOGY, HARVARD UNIVERSITY • 11 DIVINITY AVENUE CAMBRIDGE, MA 02138 DIGGING HARVARD YARD No smoking, drinking, glass-break the course, which was ing-what? Far from being a puritani taught by William Fash, cal haven, early Harvard College was a Howells director of the colorful and lively place. The first Peabody Museum, and Harvard students did more than wor Patricia Capone and Diana ship and study: they smoked, drank, Loren, Peabody Museum and broke a lot of windows. None of associate curators. They which was allowed, except on rare were assisted by Molly occasion. In the early days, Native Fierer-Donaldson, graduate American and English youth also stud student in Archaeology, ied, side by side. Archaeological and and Christina Hodge, historical records of early Harvard Peabody Museum senior Students excavating outside Matthews Hall. Photo by Patricia bring to life the experiences of our curatorial assistant and Capor1e. predecessors, teaching us that not only graduate student in is there an untold story to be Archaeology at Boston unearthed, but also, that we may learn University. ALEXANDER MARSHACK new perspectives by reflecting on our Archaeological data recovered from ARCHIVE DONATED TO THE shared history: a history that reaches Harvard Yard enriches our view of the PEABODY MUSEUM beyond the university walls to local seventeenth- through nineteenth-cen Native American communities. tury lives of students and faculty Jiving The Peabody Museum has received the The Fall 2005 course Anthropology in Harvard Yard. In the seventeenth generous donation of the photographs 1130: The Archaeology of Harvard Yard century, Harvard Yard included among and papers of Alexander Marshack brought today's Harvard students liter its four buildings the Old College, the from his widow, Elaine. -

S O C I E T Y O F S O U T H E R N C a L I F O R N
May / June 2019May METALSOCIETY OF SOUTHERN ARTS CALIFORNIA Elise Preiss MASSC Member Spotlight PRESIDENT’S MESSAGE EDITOR’S NOTES Angelina Smith John Lemieux Rose Springtime Greetings! It’s a very exciting time of year for us Metalsmiths as there are so many upcoming events and fun times. I hope everybody had a chance to finish their entries for the 2019 Metals Challenge. The deadline was April 26th, Part of the mission of The Metal so if you didn’t finish in time, you aren’t alone. I didn’t finish either. But I Arts Society of Southern Califor- still can’t wait to see the pieces that did get completed in time. They will nia is promoting our members all be on display at the Metals Challenge Luncheon on June 2nd. Thats’s and work of Southern California right! This year’s Luncheon is JUST AROUND THE CORNER! June 2nd, artists. Of course, we do that 10:00 am - 2:30 pm in Westminster. Be sure to get your tickets. We will here in our newsletter, and I’ll take the opportunity to be announcing the winner of the Nancy Monkman “Jewelry Arts Dream” invite you to be one of our Featured Artists. Just email Scholarship. The Nancy Monkman Estate Sale was well attended, thanks me a couple of photos of your work to everybody who came and purchased things, and bigger thanks to the ( [email protected] ), and I’ll take it from there. folks who spent time helping to sort, price and organize the sale. You can also show us what you are up to on Insta- Very happy to report that we conducted another successful downtown jewelry district tour this year.