Industrial Process Industrial Process
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Society, Materials, and the Environment: the Case of Steel
metals Review Society, Materials, and the Environment: The Case of Steel Jean-Pierre Birat IF Steelman, Moselle, 57280 Semécourt, France; [email protected]; Tel.: +333-8751-1117 Received: 1 February 2020; Accepted: 25 February 2020; Published: 2 March 2020 Abstract: This paper reviews the relationship between the production of steel and the environment as it stands today. It deals with raw material issues (availability, scarcity), energy resources, and generation of by-products, i.e., the circular economy, the anthropogenic iron mine, and the energy transition. The paper also deals with emissions to air (dust, Particulate Matter, heavy metals, Persistant Organics Pollutants), water, and soil, i.e., with toxicity, ecotoxicity, epidemiology, and health issues, but also greenhouse gas emissions, i.e., climate change. The loss of biodiversity is also mentioned. All these topics are analyzed with historical hindsight and the present understanding of their physics and chemistry is discussed, stressing areas where knowledge is still lacking. In the face of all these issues, technological solutions were sought to alleviate their effects: many areas are presently satisfactorily handled (the circular economy—a historical’ practice in the case of steel, energy conservation, air/water/soil emissions) and in line with present environmental regulations; on the other hand, there are important hanging issues, such as the generation of mine tailings (and tailings dam failures), the emissions of greenhouse gases (the steel industry plans to become carbon-neutral by 2050, at least in the EU), and the emission of fine PM, which WHO correlates with premature deaths. Moreover, present regulatory levels of emissions will necessarily become much stricter. -

Higher-Quality Electric-Arc Furnace Steel
ACADEMIC PULSE Higher-Quality Electric-Arc Furnace Steel teelmakers have traditionally viewed Research Continues to Improve the electric arc furnaces (EAFs) as unsuitable Quality of Steel for producing steel with the highest- Even with continued improvements to the Squality surface finish because the process design of steelmaking processes, the steelmaking uses recycled steel instead of fresh iron. With over research community has focused their attention 100 years of processing improvements, however, on the fundamental materials used in steelmaking EAFs have become an efficient and reliable in order to improve the quality of steel. In my lab steelmaking alternative to integrated steelmaking. In at Carnegie Mellon University, we have several fact, steel produced in a modern-day EAF is often research projects that deal with controlling the DR. P. BILLCHRIS MAYER PISTORIUS indistinguishable from what is produced with the impurity concentration and chemical quality of POSCOManaging Professor Editorof Materials integrated blast-furnace/oxygen-steelmaking route. steel produced in EAFs. Science412-306-4350 and Engineering [email protected] Mellon University Improvements in design, coupled with research For example, we recently used mathematical developments in metallurgy, mean high-quality steel modeling to explore ways to control produced quickly and energy-efficiently. phosphorus. Careful regulation of temperature, slag and stirring are needed to produce low- Not Your (Great-) Grandparent’s EAF phosphorus steel. We analyzed data from Especially since the mid-1990s, there have been operating furnaces and found that, in many significant improvements in the design of EAFs, cases, the phosphorus removal reaction could which allow for better-functioning burners and a proceed further. -

Petrology of Ore Deposits
Petrology of Ore Deposits An Introduction to Economic Geology Introductory Definitions Ore: a metalliferous mineral, or aggregate mixed with gangue that can me mined for a profit Gangue: associated minerals in ore deposit that have little or no value. Protore: initial non-economic concentration of metalliferous minerals that may be economic if altered by weathering (Supergene enrichment) or hydrothermal alteration Economic Considerations Grade: the concentration of a metal in an ore body is usually expressed as a weight % or ppm. The process of determining the grade is termed “assaying” Cut-off grade: after all economic and political considerations are weighed this is the lowest permissible grade that will mined. This may change over time. Example Economic Trends Economy of Scale As ore deposits are mined the high-grade zones are developed first leaving low-grade ores for the future with hopefully better technology Since mining proceeds to progressively lower grades the scale of mining increases because the amount of tonnage processed increases to remove the same amount of metal Outputs of 40,000 metric tons per day are not uncommon Near-surface open pit mines are inherently cheaper than underground mines Other factors important to mining costs include transportation, labor, power, equipment and taxation costs Classification of Ore bodies Proved ore: ore body is so thoroughly studied and understood that we can be certain of its geometry, average grade, tonnage yield, etc. Probable ore: ore body is somewhat delineated by surface mapping and some drilling. The geologists is reasonably sure of geometry and average grade. Possible Ore: outside exploration zones the geologist may speculate that the body extends some distance outside the probable zone but this is not supported by direct mapping or drilling. -

National Register of Historic Places Multiple Property
NFS Form 10-900-b 0MB No. 1024-0018 (Jan. 1987) United States Department of the Interior National Park Service National Register of Historic Places Multipler Propertyr ' Documentation Form NATIONAL This form is for use in documenting multiple property groups relating to one or several historic contexts. See instructions in Guidelines for Completing National Register Forms (National Register Bulletin 16). Complete each item by marking "x" in the appropriate box or by entering the requested information. For additional space use continuation sheets (Form 10-900-a). Type all entries. A. Name of Multiple Property Listing ____Iron and Steel Resources of Pennsylvania, 1716-1945_______________ B. Associated Historic Contexts_____________________________ ~ ___Pennsylvania Iron and Steel Industry. 1716-1945_________________ C. Geographical Data Commonwealth of Pennsylvania continuation sheet D. Certification As the designated authority under the National Historic Preservation Act of 1966, as amended, J hereby certify that this documentation form meets the National Register documentation standards and sets forth requirements for the listing of related properties consistent with the National Register criteria. This submission meets the procedural and professional requiremerytS\set forth iri36JCFR PafrfsBOfcyid the Secretary of the Interior's Standards for Planning and Evaluation. Signature of certifying official Date / Brent D. Glass Pennsylvania Historical & Museum Commission State or Federal agency and bureau I, hereby, certify that this multiple -

Three Hundred Years of Assaying American Iron and Iron Ores
Bull. Hist. Chem, 17/18 (1995) 41 THREE HUNDRED YEARS OF ASSAYING AMERICAN IRON AND IRON ORES Kvn K. Oln, WthArt It can reasonably be argued that of all of the industries factors were behind this development; increased pro- that made the modern world possible, iron and steel cess sophistication, a better understanding of how im- making holds a pivotal place. Without ferrous metals purities affected iron quality, increased capital costs, and technology, much of the modem world simply would a generation of chemically trained metallurgists enter- not exist. As the American iron industry grew from the ing the industry. This paper describes the major advances isolated iron plantations of the colonial era to the com- in analytical development. It also describes how the plex steel mills of today, the science of assaying played 19th century iron industry serves as a model for the way a critical role. The assayer gave the iron maker valu- an expanding industry comes to rely on analytical data able guidance in the quest for ever improving quality for process control. and by 1900 had laid down a theoretical foundation for the triumphs of steel in our own century. 1500's to 1800 Yet little is known about the assayer and how his By the mid 1500's the operating principles of assay labo- abilities were used by industry. Much has been written ratories were understood and set forth in the metallurgi- about the ironmaster and the furnace workers. Docents cal literature. Agricola's Mtll (1556), in period dress host historic ironmaking sites and inter- Biringuccio's rthn (1540), and the pret the lives of housewives, miners, molders, clerks, rbrbühln (Assaying Booklet, anon. -

Henry Bessemer and the Mass Production of Steel
Henry Bessemer and the Mass Production of Steel Englishmen, Sir Henry Bessemer (1813-1898) invented the first process for mass-producing steel inexpensively, essential to the development of skyscrapers. Modern steel is made using technology based on Bessemer's process. Bessemer was knighted in 1879 for his contribution to science. The "Bessemer Process" for mass-producing steel, was named after Bessemer. Bessemer's famous one-step process for producing cheap, high-quality steel made it possible for engineers to envision transcontinental railroads, sky-scraping office towers, bay- spanning bridges, unsinkable ships, and mass-produced horseless carriages. The key principle is removal of impurities from the iron by oxidation with air being blown through the molten iron. The oxidation also raises the temperature of the iron mass and keeps it molten. In the U.S., where natural resources and risk-taking investors were abundant, giant Bessemer steel mills sprung up to drive the expanding nation's rise as a dominant world economic and industrial leader. Why Steel? Steel is the most widely used of all metals, with uses ranging from concrete reinforcement in highways and in high-rise buildings to automobiles, aircraft, and vehicles in space. Steel is more ductile (able to deform without breakage) and durable than cast iron and is generally forged, rolled, or drawn into various shapes. The Bessemer process revolutionized steel manufacture by decreasing its cost. The process also decreased the labor requirements for steel-making. Prior to its introduction, steel was far too expensive to make bridges or the framework for buildings and thus wrought iron had been used throughout the Industrial Revolution. -

Principles of Extractive Metallurgy Lectures Note
PRINCIPLES OF EXTRACTIVE METALLURGY B.TECH, 3RD SEMESTER LECTURES NOTE BY SAGAR NAYAK DR. KALI CHARAN SABAT DEPARTMENT OF METALLURGICAL AND MATERIALS ENGINEERING PARALA MAHARAJA ENGINEERING COLLEGE, BERHAMPUR DISCLAIMER This document does not claim any originality and cannot be used as a substitute for prescribed textbooks. The information presented here is merely a collection by the author for their respective teaching assignments as an additional tool for the teaching-learning process. Various sources as mentioned at the reference of the document as well as freely available material from internet were consulted for preparing this document. The ownership of the information lies with the respective author or institutions. Further, this document is not intended to be used for commercial purpose and the faculty is not accountable for any issues, legal or otherwise, arising out of use of this document. The committee faculty members make no representations or warranties with respect to the accuracy or completeness of the contents of this document and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. BPUT SYLLABUS PRINCIPLES OF EXTRACTIVE METALLURGY (3-1-0) MODULE I (14 HOURS) Unit processes in Pyro metallurgy: Calcination and roasting, sintering, smelting, converting, reduction, smelting-reduction, Metallothermic and hydrogen reduction; distillation and other physical and chemical refining methods: Fire refining, Zone refining, Liquation and Cupellation. Small problems related to pyro metallurgy. MODULE II (14 HOURS) Unit processes in Hydrometallurgy: Leaching practice: In situ leaching, Dump and heap leaching, Percolation leaching, Agitation leaching, Purification of leach liquor, Kinetics of Leaching; Bio- leaching: Recovery of metals from Leach liquor by Solvent Extraction, Ion exchange , Precipitation and Cementation process. -

Iron, Steel and Swords Script - Page 1 Powder Is Difficult
10.4. Crucible Steel 10.4.1 The Making of Crucible Steel in Antiquity Debunking the Myth of "Wootz" Before I start on "wootz" I want to be very clear on how I see this rather confused topic: There never has been a secret about the making of crucible (or "wootz") steel, and there isn't one now. Recipes for making crucible steel were well-known throughout the ages, and they were just as sensible or strange as recipes for making bloomery iron or other things. There are many ways for making crucible steel. If the crucible process worked properly, the steel produced was fully liquid, and it didn't matter much exactly how you made it. If the process did not work well and the steel was only partially molten, i.e. a mixture of liquid cast iron and solid austenite, the product was not good steel. Besides differences in the (always large) carbon concentration, the concentration of impurities might also be different - just like for bloomery steel. This might lead to differences in properties for comparable carbon concentrations, for example the susceptibility to cold shortness or the ability to form a good "watered silk" or "water" pattern. Crucible steel that has been liquid once is relatively homogeneous and slag-free - in contrast to bloomery steel. That is where crucible steel is "better" than bloomery steel. Crucible steel is always high carbon if not ultra-high carbon steel (UHCS). This is generally not so good. However, mixtures that would, for example, have produced 0.8 % carbon steel, a steel optimal for many applications, would not melt at the temperatures available. -
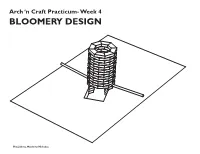
Bloomery Design
Arch ‘n Craft Practicum- Week 4 BLOOMERY DESIGN Hiu, Julieta, Matthew, Nicholas 7 3/4" The Stack: 3 3/4" In our ideal model we would use refractory bricks, either rectangular bricks or curved bricks which would help us make a circular 1'-8" stack. The stack would be around 3 feet tall with a 1 foot diameter for the inner wall of the stack. There will be openings about 3 brick lengths up(6-9 inches) from the ground on 1'-0" opposite ends of the stack. Attached to these openings will be our tuyeres which in turn will connect to our air sources. Building a circular stack would be beneficial to the type of air distribution we want to create within our furnace; using two angled tuyeres we would like air to move clockwise and counterclock- Bloomery Plan wise. We would like to line the inside of the stack with clay to provide further insulation. Refractory bricks would be an ideal material to use because the minerals they are com- posed of do not change chemically when 1'-0" exposed to high heat, some examples of these materials are aluminum, manganese, silica and zirconium. Other comments: 3'-0" Although we have opted to use refractory bricks, which would be practical for our intended purpose, we briefly considered using Vycor glass which can withstand high tem- peratures of up to 900 degrees celsius or approximately 1652 degrees Fahrenheit, which Tuyere height: Pitched -10from horizontal 7" would be enough for our purposes. Bloomery Section Ore/ Charcoal: Our group has decided to use Magnetite ore along with charcoal for the smelt. -

There's Metal in Those Hills Sheffield Was Once the Iron, Steel and Cutlery
There’s Metal in Those Hills Sheffield was once the iron, steel and cutlery capital of the world. The hills around Sheffield and Rotherham were full of raw materials like coal and iron ore that could be used in cutlery and blade production. Before we were Famous Sheffield and Rotherham became famous for manufacturing and production for four main reasons: crucible steel, stainless steel, electroplating and cutlery. Crucible Steel Benjamin Huntsman invented the crucible steel process. Before this process was invented, the quality of the steel was unreliable and slow to produce. Benjamin Huntsman’s invention allowed people to produce tougher, high-quality steel in larger quantities. Stainless Steel Harry Brearley began testing why rifles rusted and exploring ways to stop steel from rusting. He developed Stainless Steel which was much more rust resistant than the steel that had been used previously. Cutlery Sheffield and Rotherham have been connected to the production of cutlery since the 1600s. Sheffield was the main center of cutlery production in England outside of London. Sheffield became world famous for its production of high-quality cutlery. The moving story of Sheffield’s remarkable Women of Steel Yorkshire Post article 13th June 2020 The story of Kathleen, one of the last surviving Sheffield steelworkers from the war. When war broke out, the lives of the young women of Sheffield were turned upside down. With the men sent away to fight they had no choice but to step into their shoes and became the backbone of the city’s steel industry. Through hard graft and companionship in the gruelling, and often dangerous world of factory work, they vowed to keep the foundry fires burning. -

Experimental Design for Copper Cementation Process in Fixed Bed
Arabian Journal of Chemistry (2010) 3, 187–190 King Saud University Arabian Journal of Chemistry www.ksu.edu.sa www.sciencedirect.com ORIGINAL ARTICLE Experimental design for copper cementation process in fixed bed reactor using two-level factorial design I. Yahiaoui *, F. Aissani-Benissad Laboratoire de Ge´nie de l’Environnement (LGE), De´partement de Ge´nie des Proce´de´s, Faculte´ de la Technologie, Universite´ A.MIRA de Bejaia 06000, Algeria Received 20 July 2009; accepted 20 December 2009 Available online 18 April 2010 KEYWORDS Abstract This work deals with cementation of copper onto iron grid in a fixed bed reactor. The 2+ Copper; influence of several parameters is studied, namely: initial concentration of copper [Cu ]0, temper- Iron; ature and flow rate. Moreover, their influence on the copper cementation reaction is investigated Fixed bed reactor; statistically by the experimental design in view of industrial application. The estimation and the Factorial design comparison of the parameter’s effects are realized by using two-level factorial design. The analysis of these effects permits to state that the most influential factor is initial concentration of copper 2+ [Cu ]0 with an effect of (+2.4566), the second in the order is the temperature with an effect of (+0.18959), the third is the flow rate of the electrolytic solution with an effect of (À0.4226). The significance interactions found by the design of experiments are between initial concentrations of copper ions–flow rate (x1x3) with an effect (b13 = +0.6965). ª 2010 King Saud University. All rights reserved. 1. Introduction Ultimately, this wastewater is discharged in permitted concen- trations of suspended solids and dissolved salts. -

Master's Thesis
MASTER'S THESIS Energy System Analysis in the Swedish Iron and Steel Industry Ernesto Ubieto Master of Science (120 credits) Mechanical Engineering Luleå University of Technology Department of Engineering Sciences and Mathematics Energy System Analysis of the Swedish Iron and Steel Industry Ernesto Ubieto Udina Table of contents 1 INTRODUCTION ................................................................................................................. 7 2 OBJECTIVES ....................................................................................................................... 8 3 METHODOLOGY ................................................................................................................ 9 3.1 Methodology of System Analysis ............................................................................... 9 3.1.1 Scope of the Analysis ....................................................................................... 10 3.1.2 Boundaries of the Analysis ............................................................................... 11 3.1.3 Time frames ..................................................................................................... 11 3.1.4 Components of the System .............................................................................. 12 3.1.5 Connections within the system ........................................................................ 13 3.1.6 Limitations of the study ................................................................................... 15 3.1.7 Tracking CO2