Isolation and Structure of Two Prostaglandin Endoperoxides That
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Aryl Hydrocarbon Receptor: Structural Analysis and Activation Mechanisms
The Aryl Hydrocarbon Receptor: Structural Analysis and Activation Mechanisms This thesis is submitted in fulfilment of the requirements for the degree of Doctor of Philosophy in the School of Molecular and Biomedical Sciences (Biochemistry), The University of Adelaide, Australia Fiona Whelan, B.Sc. (Hons) 2009 2 Table of Contents THESIS SUMMARY................................................................................. 6 DECLARATION....................................................................................... 7 PUBLICATIONS ARISING FROM THIS THESIS.................................... 8 ACKNOWLEDGEMENTS...................................................................... 10 ABBREVIATIONS ................................................................................. 12 CHAPTER 1: INTRODUCTION ............................................................. 17 1.1 BHLH.PAS PROTEINS ............................................................................................17 1.1.1 General background..................................................................................17 1.1.2 bHLH.PAS Class I Proteins.........................................................................18 1.2 THE ARYL HYDROCARBON RECEPTOR......................................................................19 1.2.1 Domain Structure and Ligand Activation ..............................................19 1.2.2 AhR Expression and Developmental Activity .......................................21 1.2.3 Mouse AhR Knockout Phenotype ...........................................................23 -

AA Metabolism, 139 A2b1, 69, 70, 318–323 ABCB1 Gene, 176 Abciximab
Index A aIIbb3, 60, 62, 69, 70, 73, 74, 79, 80 AA. See Arachidonic acid (AA) AJW202, 293 AA metabolism, 139 Akt-1, 345 a2b1, 69, 70, 318–323 Akt-2, 345 ABCB1 gene, 176 ALX-0081, 296 Abciximab, 206–208, 497, 500, 501, 504–505, ALX-0681, 298 509, 513, 514, 525 Anagrelide, 227, 229 approval, 206 Ankle brachial indexes (ABIs), 549, 587, EPIC trial, 207 595–597 EPILOG study, 207 framingham risk score, 551 EPISTENT study, 207 screening, 550 platelet binding, 207 sensitivity, 551 thrombocytopenia, 206 specificity, 551 in unstable angina, 208 vascular risk, 550 ABIs. See Ankle brachial indexes (ABIs) Antagomirs, 439 Acetylsalicylic acid (aspirin), 137–158 Antibodies, 317 ACS. See Acute coronary syndromes (ACS) Anticoagulants, 525, 537 Acute coronary syndromes (ACS), 288 Anticoagulation, 533, 534 Acute ischemic stroke, 143, 149–150 Antioxidant, 233 Acute myocardial infarction, 142, 143, 149, Antiplatelet agents, 263, 553 153, 157 Antithrombotic Trialist’s ADAMTS-13, 93 Collaboration, 553 Adenosine diphosphate (ADP), 37, 88, 96, aspirin, 553 166, 473 clopidogrel, 553 Adenosine triphosphate (ATP), 37 dipyridamole, 553 Adhesion, 90, 112–118, 125 meta-analysis, 560 Adhesion molecules infiltration, 264 picotamide, 553 Adiponectin, 315 risk reduction, 553 ADP. See Adenosine diphosphate (ADP) ticlopidine, 553 ADP inhibitors (or P2Y12 blockers), 497–499, vascular events, 553 501–505, 507–509, 513, 514 Antiplatelet therapy, 472 ADP-receptor antagonists, 169 Apixaban, 535, 539 Adverse effects, 153–156 Aptamers, 299–301 Aegyptin, 327 Arachidonic acid (AA), 474 P. Gresele et al. (eds.), Antiplatelet Agents, Handbook of Experimental 607 Pharmacology 210, DOI 10.1007/978-3-642-29423-5, # Springer-Verlag Berlin Heidelberg 2012 608 Index ARC1779, 299–300 TXS signaling, 279 ARC15105, 300 Cardiovascular death, 248 AR-C69931MX, 456 Carotid endarterectomy (ACE) inhibition, Aspirin, 474, 496, 498–499, 501–502, 143, 156 504–507, 511–514, 520, 522, Carotid stenosis, 523 527, 531, 533–537, 539, CCBs. -

Open Thesis Master Document V5.0.Pdf
The Pennsylvania State University The Graduate School Department of Veterinary and Biomedical Science IDENTIFICATION OF ENDOGENOUS MODULATORS FOR THE ARYL HYDROCARBON RECEPTOR A Thesis in Genetics by Christopher R. Chiaro © 2007 Christopher R. Chiaro Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December, 2007 The thesis of Christopher R. Chiaro was reviewed and approved* by the following: Gary H. Perdew John T. and Paige S. Smith Professor in Agricultural Sciences Thesis Advisor Chair of Committee C. Channa Reddy Distinguished Professor of Veterinary Science A. Daniel Jones Senior Scientist Department of Chemistry John P. Vanden Heuvel Professor of Veterinary Science Richard Ordway Associate Professor of Biology Chair of Genetics Graduate Program *Signatures are on file in the Graduate School iii ABSTRACT The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor capable of being regulated by a structurally diverse array of chemicals ranging from environmental carcinogens to dietary metabolites. A member of the basic helix-loop- helix/ Per-Arnt-Sim (bHLH-PAS) super-family of DNA binding regulatory proteins, the AhR is an important developmental regulator that can be detected in nearly all mammalian tissues. Prior to ligand activation, the AhR resides in the cytosol as part of an inactive oligomeric protein complex comprised of the AhR ligand-binding subunit, a dimer of the 90 kDa heat shock protein, and a single molecule each of the immunophilin like X-associated protein 2 (XAP2) and p23 proteins. Functioning as chemosensor, the AhR responds to both endobiotic and xenobiotic derived chemical ligands by ultimately directing the expression of metabolically important target genes. -

Epigenetic Regulations of Ahr in the Aspect of Immunomodulation
International Journal of Molecular Sciences Review Epigenetic Regulations of AhR in the Aspect of Immunomodulation Anna Wajda 1,* , Joanna Łapczuk-Roma ´nska 2 and Agnieszka Paradowska-Gorycka 1 1 Department of Molecular Biology, National Institute of Geriatrics, Rheumatology and Rehabilitation, 02-637 Warsaw, Poland; [email protected] 2 Department of Experimental and Clinical Pharmacology, Pomeranian Medical University, 70-111 Szczecin, Poland; [email protected] * Correspondence: [email protected] Received: 31 July 2020; Accepted: 28 August 2020; Published: 3 September 2020 Abstract: Environmental factors contribute to autoimmune disease manifestation, and as regarded today, AhR has become an important factor in studies of immunomodulation. Besides immunological aspects, AhR also plays a role in pharmacological, toxicological and many other physiological processes such as adaptive metabolism. In recent years, epigenetic mechanisms have provided new insight into gene regulation and reveal a new contribution to autoimmune disease pathogenesis. DNA methylation, histone modifications, chromatin alterations, microRNA and consequently non-genetic changes in phenotypes connect with environmental factors. Increasing data reveals AhR cross-roads with the most significant in immunology pathways. Although study on epigenetic modulations in autoimmune diseases is still not well understood, therefore future research will help us understand their pathophysiology and help to find new therapeutic strategies. Present literature review -

Role of Arachidonic Acid and Its Metabolites in the Biological and Clinical Manifestations of Idiopathic Nephrotic Syndrome
International Journal of Molecular Sciences Review Role of Arachidonic Acid and Its Metabolites in the Biological and Clinical Manifestations of Idiopathic Nephrotic Syndrome Stefano Turolo 1,* , Alberto Edefonti 1 , Alessandra Mazzocchi 2, Marie Louise Syren 2, William Morello 1, Carlo Agostoni 2,3 and Giovanni Montini 1,2 1 Fondazione IRCCS Ca’ Granda-Ospedale Maggiore Policlinico, Pediatric Nephrology, Dialysis and Transplant Unit, Via della Commenda 9, 20122 Milan, Italy; [email protected] (A.E.); [email protected] (W.M.); [email protected] (G.M.) 2 Department of Clinical Sciences and Community Health, University of Milan, 20122 Milan, Italy; [email protected] (A.M.); [email protected] (M.L.S.); [email protected] (C.A.) 3 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Pediatric Intermediate Care Unit, 20122 Milan, Italy * Correspondence: [email protected] Abstract: Studies concerning the role of arachidonic acid (AA) and its metabolites in kidney disease are scarce, and this applies in particular to idiopathic nephrotic syndrome (INS). INS is one of the most frequent glomerular diseases in childhood; it is characterized by T-lymphocyte dysfunction, alterations of pro- and anti-coagulant factor levels, and increased platelet count and aggregation, leading to thrombophilia. AA and its metabolites are involved in several biological processes. Herein, Citation: Turolo, S.; Edefonti, A.; we describe the main fields where they may play a significant role, particularly as it pertains to their Mazzocchi, A.; Syren, M.L.; effects on the kidney and the mechanisms underlying INS. AA and its metabolites influence cell Morello, W.; Agostoni, C.; Montini, G. -
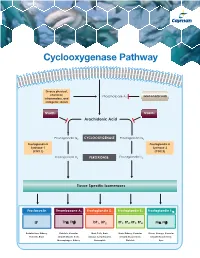
Cyclooxygenase Pathway
Cyclooxygenase Pathway Diverse physical, chemical, Phospholipase A Glucocorticoids inflammatory, and 2 mitogenic stimuli NSAIDs NSAIDs Arachidonic Acid Prostaglandin G2 CYCLOOXYGENASE Prostaglandin G2 Prostaglandin H Prostaglandin H Synthase-1 Synthase-2 (COX 1) (COX 2) Prostaglandin H2 PEROXIDASE Prostaglandin H2 Tissue Specific Isomerases Prostacyclin Thromboxane A2 Prostaglandin D2 Prostaglandin E2 Prostaglandin F2α IP TPα, TPβ DP1, DP2 EP1, EP2, EP3, EP4 FPα, FPβ Endothelium, Kidney, Platelets, Vascular Mast Cells, Brain, Brain, Kidney, Vascular Uterus, Airways, Vascular Platelets, Brain Smooth Muscle Cells, Airways, Lymphocytes, Smooth Muscle Cells, Smooth Muscle Cells, Macrophages, Kidney Eosinophils Platelets Eyes Prostacyclin Item No. Product Features Prostacyclin (Prostaglandin I2; PGI2) is formed from arachidonic acid primarily in the vascular endothelium and renal cortex by sequential 515211 6-keto • Sample Types: Culture Medium | Plasma Prostaglandin • Measure 6-keto PGF levels down to 6 pg/ml activities of COX and prostacyclin synthase. PGI2 is non-enzymatically 1α F ELISA Kit • Incubation : 18 hours | Development: 90-120 minutes | hydrated to 6-keto PGF1α (t½ = 2-3 minutes), and then quickly converted 1α Read: Colorimetric at 405-420 nm to the major metabolite, 2,3-dinor-6-keto PGF1α (t½= 30 minutes). Prostacyclin was once thought to be a circulating hormone that regulated • Assay 24 samples in triplicate or 36 samples in duplicate platelet-vasculature interactions, but the rate of secretion into circulation • NOTE: A portion of urinary 6-keto PGF1α is of renal origin coupled with the short half-life indicate that prostacyclin functions • NOTE : It has been found that normal plasma levels of 6-keto PGF may be low locally. -

Effect of Prostanoids on Human Platelet Function: an Overview
International Journal of Molecular Sciences Review Effect of Prostanoids on Human Platelet Function: An Overview Steffen Braune, Jan-Heiner Küpper and Friedrich Jung * Institute of Biotechnology, Molecular Cell Biology, Brandenburg University of Technology, 01968 Senftenberg, Germany; steff[email protected] (S.B.); [email protected] (J.-H.K.) * Correspondence: [email protected] Received: 23 October 2020; Accepted: 23 November 2020; Published: 27 November 2020 Abstract: Prostanoids are bioactive lipid mediators and take part in many physiological and pathophysiological processes in practically every organ, tissue and cell, including the vascular, renal, gastrointestinal and reproductive systems. In this review, we focus on their influence on platelets, which are key elements in thrombosis and hemostasis. The function of platelets is influenced by mediators in the blood and the vascular wall. Activated platelets aggregate and release bioactive substances, thereby activating further neighbored platelets, which finally can lead to the formation of thrombi. Prostanoids regulate the function of blood platelets by both activating or inhibiting and so are involved in hemostasis. Each prostanoid has a unique activity profile and, thus, a specific profile of action. This article reviews the effects of the following prostanoids: prostaglandin-D2 (PGD2), prostaglandin-E1, -E2 and E3 (PGE1, PGE2, PGE3), prostaglandin F2α (PGF2α), prostacyclin (PGI2) and thromboxane-A2 (TXA2) on platelet activation and aggregation via their respective receptors. Keywords: prostacyclin; thromboxane; prostaglandin; platelets 1. Introduction Hemostasis is a complex process that requires the interplay of multiple physiological pathways. Cellular and molecular mechanisms interact to stop bleedings of injured blood vessels or to seal denuded sub-endothelium with localized clot formation (Figure1). -

2374 Supplementary Drugs and Other Substances
2374 Supplementary Drugs and Other Substances 5. Burdock GA. Review of the biological properties and toxicity of Pharmacokinetics reduced to the endoperoxide prostaglandin H2 (PGH2). Prostag- bee propolis (propolis). Food Chem Toxicol 1998; 36: 347–63. Propylene glycol is rapidly absorbed from the gastrointestinal landin H2 is then converted to the primary prostaglandins pros- 6. Lieberman HD, et al. Allergic contact dermatitis to propolis in a tract. There is evidence of topical absorption when applied to taglandin D2, prostaglandin E2, and prostaglandin F2 , to throm- violin maker. J Am Acad Dermatol 2002; 46 (suppl): S30–S31. damaged skin. boxane A2 (TXA2) via the enzyme thromboxane synthetase, or 7. Giusti F, et al. Sensitization to propolis in 1255 children under- It is extensively metabolised in the liver primarily by oxidation to prostacyclin (PGI2) via the enzyme prostacyclin synthetase. going patch testing. Contact Dermatitis 2004; 51: 255–8. to lactic and pyruvic acid and is also excreted in the urine These products are further metabolised and rapidly inactivated in 8. Walgrave SE, et al. Allergic contact dermatitis from propolis. unchanged. the body. Dermatitis 2005; 16: 209–15. The secondary prostaglandins, prostaglandin A (PGA ), pros- 9. Majiene D, et al. Antifungal and antibacterial activity of propo- ◊ References. 2 2 lis. Curr Nutr Food Sci 2007; 3: 304–8. 1. Yu DK, et al. Pharmacokinetics of propylene glycol in humans taglandin B2 (PGB2), and prostaglandin C2 (PGC2) are derived during multiple dosing regimens. J Pharm Sci 1985; 74: 876–9. from prostaglandin E2, but are formed during extraction and Preparations 2. Speth PAJ, et al. -

The Protective Effects of Conjugated Linoleic Acid Against Carcinogenesis
The Protective Effects of Conjugated Linoleic Acid Against Carcinogenesis Item Type text; Electronic Dissertation Authors Kemp, Michael Quentin Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 24/09/2021 15:13:15 Link to Item http://hdl.handle.net/10150/193637 THE PROTECTIVE EFFECTS OF CONJUGATED LINOLEIC ACID AGAINST CARCINOGENESIS by Michael Quentin Kemp A Dissertation Submitted to the Faculty of the GRADUATE PROGRAM IN NUTRITIONAL SCIENCES In Partial Fulfillment of the Requirements for the Degree of DOC TORAL OF PHILOSOPHY In the Graduate College THE UNIVERSITY OF ARIZONA 2005 2 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Michael Q. Kemp entitled The Protective Effec ts of Conjugated Linoleic Acid Against Carcinogenesis and recommend that it be accepted as fulfilling the dissertation requirement for the Deg ree of Doctor of Philosophy _______ ___________________________ __________________ ________ _______ ___ _ Date : 11/18/05 . Donato Romagnolo _______ ___________________________ __________________ ________ ___________ Date: 11/18/05 . Wanda Howell _______ ___________________________ __________________ ________ ___________ Date: 11/18/05 . Linda Houtkooper _______ ___________________________ __________________ ________ ___________ Date: 11/18/05 . Scott Going _______ ___________________________ __________________ ________ ___________ Date: 11/18/05 . Joy Winzerling Final approval and acceptance of this dissertation is contingent upon the candidate’s submission of the final copies of the dissertation to the Graduate College. -

COX-1 and COX-2 Enzymes Synthesize Prostaglandins and Are Teacher Emeritus, University of Wisconsin-Madison) Mentor: Dr
COX-1 And COX-2 Enzymes Synthesize Prostaglandins and Are Teacher Emeritus, University of Wisconsin-Madison) Mentor: Dr. David Nelson (Professor of Biochemistry, University of Wisconsin- (Student, University of Wisconsin-Madison) Center for Inhibited by NSAIDS (Nonsteroidal Anti-inflammatory Drugs) BioMolecular Madison West High School: Audra Amasino, Yuting Deng, Samuel Huang, Iris Lee, Adeyinka Lesi, Yaoli Pu, and Peter Vander Velden Modeling Advisor: Gary Graper, Teacher Emeritus, University of Wisconsin-Madison Mentors: Dr. David Nelson, Professor of Biochemistry, and Basudeb Bhattacharyya, Student, University of Wisconsin-Madison Abstract Prostaglandin Hormone Synthases (COX-1 and COX-2) are enzymes that produce prostaglandins. Prostaglandins are (1) Structure (3) Cyclooxygenase Active Sites responsible for fever, pain, and inflammation, but also the (5) Drugs maintenance of the lining of the stomach and prevention of In the pictures below, the heme is orange, the ulceration. COX-1 is found mainly in the gastrointestinal lining, hydrophobic knob is yellow, and the amino acids in the The three drugs below are all nonsteroidal anti- and COX-2 at sites of inflammation. NSAIDS (Nonsteroidal anti- Cyclooxygenase active site are colored in CPK (red for inflammatory drugs (NSAIDS). The first two NSAIDS, inflammatory drugs) such as aspirin, ibuprofen, naproxen, and oxygen, blue for nitrogen, and gray for carbon). aspirin and ibuprofen, are called nonselective Cox flurbiprofen inhibit both COX-1 and COX-2, and are taken inhibitors since they affect both COX-1 and COX-2 regularly by over 33 million Americans for pain and substantially (note their high COX-2/COX-1 effect inflammation. Some 10%-50% of these users suffer ratios). -

Effects of Prostaglandin H2 on Perinatal Pulmonary Circulation
003 1-3998/86/2006-0565$02.00/0 PEDIATRIC RESEARCH Vol. 20, No. 6, 1986 Copyright O 1986 International Pediatric Research Foundation, Inc. Printed in U.S.A. Effects of Prostaglandin H2 on Perinatal Pulmonary Circulation MARY L. TOD,' SIDNEY CASSIN, DENNIS B. McNAMARA, AND PHILIP J. KADOWITZ Departments of Physiology and Pediatrics, College of Medicine, University of Florida, Gainesville, Florida 32610 and Department of Pharmacology, Tulane Medical School, New Orleans, Louisiana 70112 ABSTRACT. A pivotal intermediate in prostaglandin (PG) terminal enzymes that yield these various products are present biosynthesis is the endoperoxide PGH2. This endoperox- in varying amounts and activities in different organs, and this ide is capable of eliciting direct responses in biological distribution contributes to the wide range of responses of indi- systems without undergoing conversion to other PGs. Ef- vidual systems to release of PGs (1-6). There is evidence that fects of PGH2 include stimulation of platelet aggregation PGH2 is capable of eliciting direct responses to biological systems and vascular smooth muscle contraction in vitro; injections without undergoing conversion to other PGs (7, 8). Kadowitz et of PGH2 in vivo cause increases in pulmonary arterial al. (9) demonstrated the ability of PGH2 to contract isolated pressure. The response of the pulmonary vasculature of segments of canine intrapulmonary vein. Additionally, bolus perinatal lambs to PGH2 was measured using an in situ injections of PGH2 caused increases in lobar arterial and small pump-perfused left lower lung preparation. Intrapulmonary vein pressures in pump-perfused canine lungs (9). Unanes- injections of PGH2 (0.24-0.61 pg/kg) into six unventilated thetized adult sheep responded to infusions of PGH2 with dose- fetal lambs (0.93-0.97 gestation) produced decreases in related increases of pulmonary arterial pressure (10). -

University of Groningen Reflections on Flurbiprofen Eyedrops Van Sorge
University of Groningen Reflections on flurbiprofen eyedrops van Sorge, Adriaan Alastair IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2002 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): van Sorge, A. A. (2002). Reflections on flurbiprofen eyedrops. s.n. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 25-09-2021 REFLECTIONS ON FLURBIPROFEN EYEDROPS REFLECTIONS ON FLURBIPROFEN EYEDROPS RIJKSUNIVERSITEIT GRONINGEN REFLECTIONS ON FLURBIPROFEN EYEDROPS REFLECTIONS ON FLURBIPROFEN EYEDROPS PROEFSCHRIFT ter verkrijging van het doctoraat in de Wiskunde en Natuurwetenschappen aan de Rijksuniversiteit Groningen, op gezag van de Rector Magnificus, dr. F. Zwarts, in het openbaar te verdedigen op maandag 2 december 2002 om 14.15 uur door Adriaan Alastair van Sorge geboren op 28 oktober 1944 te New Rochelle, New York, USA PROMOTORES Prof.