NS-398 Upregulates Constitutive Cyclooxygenase-2 Expression in the M-1 Cortical Collecting Duct Cell Line
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Aryl Hydrocarbon Receptor: Structural Analysis and Activation Mechanisms
The Aryl Hydrocarbon Receptor: Structural Analysis and Activation Mechanisms This thesis is submitted in fulfilment of the requirements for the degree of Doctor of Philosophy in the School of Molecular and Biomedical Sciences (Biochemistry), The University of Adelaide, Australia Fiona Whelan, B.Sc. (Hons) 2009 2 Table of Contents THESIS SUMMARY................................................................................. 6 DECLARATION....................................................................................... 7 PUBLICATIONS ARISING FROM THIS THESIS.................................... 8 ACKNOWLEDGEMENTS...................................................................... 10 ABBREVIATIONS ................................................................................. 12 CHAPTER 1: INTRODUCTION ............................................................. 17 1.1 BHLH.PAS PROTEINS ............................................................................................17 1.1.1 General background..................................................................................17 1.1.2 bHLH.PAS Class I Proteins.........................................................................18 1.2 THE ARYL HYDROCARBON RECEPTOR......................................................................19 1.2.1 Domain Structure and Ligand Activation ..............................................19 1.2.2 AhR Expression and Developmental Activity .......................................21 1.2.3 Mouse AhR Knockout Phenotype ...........................................................23 -

Open Thesis Master Document V5.0.Pdf
The Pennsylvania State University The Graduate School Department of Veterinary and Biomedical Science IDENTIFICATION OF ENDOGENOUS MODULATORS FOR THE ARYL HYDROCARBON RECEPTOR A Thesis in Genetics by Christopher R. Chiaro © 2007 Christopher R. Chiaro Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December, 2007 The thesis of Christopher R. Chiaro was reviewed and approved* by the following: Gary H. Perdew John T. and Paige S. Smith Professor in Agricultural Sciences Thesis Advisor Chair of Committee C. Channa Reddy Distinguished Professor of Veterinary Science A. Daniel Jones Senior Scientist Department of Chemistry John P. Vanden Heuvel Professor of Veterinary Science Richard Ordway Associate Professor of Biology Chair of Genetics Graduate Program *Signatures are on file in the Graduate School iii ABSTRACT The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor capable of being regulated by a structurally diverse array of chemicals ranging from environmental carcinogens to dietary metabolites. A member of the basic helix-loop- helix/ Per-Arnt-Sim (bHLH-PAS) super-family of DNA binding regulatory proteins, the AhR is an important developmental regulator that can be detected in nearly all mammalian tissues. Prior to ligand activation, the AhR resides in the cytosol as part of an inactive oligomeric protein complex comprised of the AhR ligand-binding subunit, a dimer of the 90 kDa heat shock protein, and a single molecule each of the immunophilin like X-associated protein 2 (XAP2) and p23 proteins. Functioning as chemosensor, the AhR responds to both endobiotic and xenobiotic derived chemical ligands by ultimately directing the expression of metabolically important target genes. -

Epigenetic Regulations of Ahr in the Aspect of Immunomodulation
International Journal of Molecular Sciences Review Epigenetic Regulations of AhR in the Aspect of Immunomodulation Anna Wajda 1,* , Joanna Łapczuk-Roma ´nska 2 and Agnieszka Paradowska-Gorycka 1 1 Department of Molecular Biology, National Institute of Geriatrics, Rheumatology and Rehabilitation, 02-637 Warsaw, Poland; [email protected] 2 Department of Experimental and Clinical Pharmacology, Pomeranian Medical University, 70-111 Szczecin, Poland; [email protected] * Correspondence: [email protected] Received: 31 July 2020; Accepted: 28 August 2020; Published: 3 September 2020 Abstract: Environmental factors contribute to autoimmune disease manifestation, and as regarded today, AhR has become an important factor in studies of immunomodulation. Besides immunological aspects, AhR also plays a role in pharmacological, toxicological and many other physiological processes such as adaptive metabolism. In recent years, epigenetic mechanisms have provided new insight into gene regulation and reveal a new contribution to autoimmune disease pathogenesis. DNA methylation, histone modifications, chromatin alterations, microRNA and consequently non-genetic changes in phenotypes connect with environmental factors. Increasing data reveals AhR cross-roads with the most significant in immunology pathways. Although study on epigenetic modulations in autoimmune diseases is still not well understood, therefore future research will help us understand their pathophysiology and help to find new therapeutic strategies. Present literature review -

Role of Arachidonic Acid and Its Metabolites in the Biological and Clinical Manifestations of Idiopathic Nephrotic Syndrome
International Journal of Molecular Sciences Review Role of Arachidonic Acid and Its Metabolites in the Biological and Clinical Manifestations of Idiopathic Nephrotic Syndrome Stefano Turolo 1,* , Alberto Edefonti 1 , Alessandra Mazzocchi 2, Marie Louise Syren 2, William Morello 1, Carlo Agostoni 2,3 and Giovanni Montini 1,2 1 Fondazione IRCCS Ca’ Granda-Ospedale Maggiore Policlinico, Pediatric Nephrology, Dialysis and Transplant Unit, Via della Commenda 9, 20122 Milan, Italy; [email protected] (A.E.); [email protected] (W.M.); [email protected] (G.M.) 2 Department of Clinical Sciences and Community Health, University of Milan, 20122 Milan, Italy; [email protected] (A.M.); [email protected] (M.L.S.); [email protected] (C.A.) 3 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Pediatric Intermediate Care Unit, 20122 Milan, Italy * Correspondence: [email protected] Abstract: Studies concerning the role of arachidonic acid (AA) and its metabolites in kidney disease are scarce, and this applies in particular to idiopathic nephrotic syndrome (INS). INS is one of the most frequent glomerular diseases in childhood; it is characterized by T-lymphocyte dysfunction, alterations of pro- and anti-coagulant factor levels, and increased platelet count and aggregation, leading to thrombophilia. AA and its metabolites are involved in several biological processes. Herein, Citation: Turolo, S.; Edefonti, A.; we describe the main fields where they may play a significant role, particularly as it pertains to their Mazzocchi, A.; Syren, M.L.; effects on the kidney and the mechanisms underlying INS. AA and its metabolites influence cell Morello, W.; Agostoni, C.; Montini, G. -
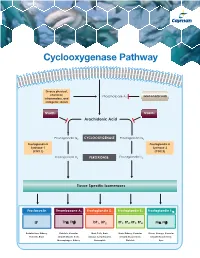
Cyclooxygenase Pathway
Cyclooxygenase Pathway Diverse physical, chemical, Phospholipase A Glucocorticoids inflammatory, and 2 mitogenic stimuli NSAIDs NSAIDs Arachidonic Acid Prostaglandin G2 CYCLOOXYGENASE Prostaglandin G2 Prostaglandin H Prostaglandin H Synthase-1 Synthase-2 (COX 1) (COX 2) Prostaglandin H2 PEROXIDASE Prostaglandin H2 Tissue Specific Isomerases Prostacyclin Thromboxane A2 Prostaglandin D2 Prostaglandin E2 Prostaglandin F2α IP TPα, TPβ DP1, DP2 EP1, EP2, EP3, EP4 FPα, FPβ Endothelium, Kidney, Platelets, Vascular Mast Cells, Brain, Brain, Kidney, Vascular Uterus, Airways, Vascular Platelets, Brain Smooth Muscle Cells, Airways, Lymphocytes, Smooth Muscle Cells, Smooth Muscle Cells, Macrophages, Kidney Eosinophils Platelets Eyes Prostacyclin Item No. Product Features Prostacyclin (Prostaglandin I2; PGI2) is formed from arachidonic acid primarily in the vascular endothelium and renal cortex by sequential 515211 6-keto • Sample Types: Culture Medium | Plasma Prostaglandin • Measure 6-keto PGF levels down to 6 pg/ml activities of COX and prostacyclin synthase. PGI2 is non-enzymatically 1α F ELISA Kit • Incubation : 18 hours | Development: 90-120 minutes | hydrated to 6-keto PGF1α (t½ = 2-3 minutes), and then quickly converted 1α Read: Colorimetric at 405-420 nm to the major metabolite, 2,3-dinor-6-keto PGF1α (t½= 30 minutes). Prostacyclin was once thought to be a circulating hormone that regulated • Assay 24 samples in triplicate or 36 samples in duplicate platelet-vasculature interactions, but the rate of secretion into circulation • NOTE: A portion of urinary 6-keto PGF1α is of renal origin coupled with the short half-life indicate that prostacyclin functions • NOTE : It has been found that normal plasma levels of 6-keto PGF may be low locally. -

The Protective Effects of Conjugated Linoleic Acid Against Carcinogenesis
The Protective Effects of Conjugated Linoleic Acid Against Carcinogenesis Item Type text; Electronic Dissertation Authors Kemp, Michael Quentin Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 24/09/2021 15:13:15 Link to Item http://hdl.handle.net/10150/193637 THE PROTECTIVE EFFECTS OF CONJUGATED LINOLEIC ACID AGAINST CARCINOGENESIS by Michael Quentin Kemp A Dissertation Submitted to the Faculty of the GRADUATE PROGRAM IN NUTRITIONAL SCIENCES In Partial Fulfillment of the Requirements for the Degree of DOC TORAL OF PHILOSOPHY In the Graduate College THE UNIVERSITY OF ARIZONA 2005 2 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Michael Q. Kemp entitled The Protective Effec ts of Conjugated Linoleic Acid Against Carcinogenesis and recommend that it be accepted as fulfilling the dissertation requirement for the Deg ree of Doctor of Philosophy _______ ___________________________ __________________ ________ _______ ___ _ Date : 11/18/05 . Donato Romagnolo _______ ___________________________ __________________ ________ ___________ Date: 11/18/05 . Wanda Howell _______ ___________________________ __________________ ________ ___________ Date: 11/18/05 . Linda Houtkooper _______ ___________________________ __________________ ________ ___________ Date: 11/18/05 . Scott Going _______ ___________________________ __________________ ________ ___________ Date: 11/18/05 . Joy Winzerling Final approval and acceptance of this dissertation is contingent upon the candidate’s submission of the final copies of the dissertation to the Graduate College. -

University of Groningen Reflections on Flurbiprofen Eyedrops Van Sorge
University of Groningen Reflections on flurbiprofen eyedrops van Sorge, Adriaan Alastair IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2002 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): van Sorge, A. A. (2002). Reflections on flurbiprofen eyedrops. s.n. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 25-09-2021 REFLECTIONS ON FLURBIPROFEN EYEDROPS REFLECTIONS ON FLURBIPROFEN EYEDROPS RIJKSUNIVERSITEIT GRONINGEN REFLECTIONS ON FLURBIPROFEN EYEDROPS REFLECTIONS ON FLURBIPROFEN EYEDROPS PROEFSCHRIFT ter verkrijging van het doctoraat in de Wiskunde en Natuurwetenschappen aan de Rijksuniversiteit Groningen, op gezag van de Rector Magnificus, dr. F. Zwarts, in het openbaar te verdedigen op maandag 2 december 2002 om 14.15 uur door Adriaan Alastair van Sorge geboren op 28 oktober 1944 te New Rochelle, New York, USA PROMOTORES Prof. -

Isolation and Structure of Two Prostaglandin Endoperoxides That
Proc. Nat. Acad. Sci. USA Vol. 71, No. 2, pp. 345-349, February 1974 Isolation and Structure of Two Prostaglandin Endoperoxides That Cause Platelet Aggregation (15-hydroperoxy endoperoxide/15-hydroxy endoperoxide/platelet aggregation/ contraction of rabbit aorta) MATS HAMBERG, JAN SVENSSON, TOSHIO WAKABAYASHI, AND BENGT SAMUELSSON Department of Chemistry, Karolinska Institutet, S 104 01, Stockholm, Sweden Communicated by Hugo Theorell, September 19, 1978 ABSTRACT Incubation for a short time of arachidonic (kindly provided by Dr. W. Stoffel, Cologne, Germany, see acid with the microsomal fraction of a homogenate of the ref. 2) and Na14CN followed by hydrolysis of the nitrile. The vesicular gland of sheep in the presence of 1 mM p-mer- curibenzoate followed by extraction and silicic acid chemical and radiochemical purity was in excess of 98%, chromatography yielded two prostaglandin endoper- as judged by thin-layer radiochromatography. Part of the oxides. The structures of these compounds, i.e., 15-hy- labeled acid was diluted with unlabeled material to make a droperoxy-9a,11a-peroxidoprosta-5,13-dienoic acid (pros- preparation with specific radioactivity of 0.77 Ci/mol, which taglandin G2) and 15-hydroxy-9a,lla-peroxidoprosta- 5,13-dienoic acid (prostaglandin H2), were assigned mainly was used for incubations with vesicular gland microsomes. by a number of chemical transformations into previously Thin-Layer Chromatography (TLC) was carried out with known prostaglandins. The new prostaglandins were 50- 200 times (prostaglandin G2) and 100-450 times (prosta- plates coated with chloroform-methanol-washed Silica gel G glandin H2) more active than prostaglandin E2 on the super- and the following solvent systems (when not otherwise fused aorta strip. -

Prostacyclin: an Inflammatory Paradox
REVIEW ARTICLE published: 13 May 2011 doi: 10.3389/fphar.2011.00024 Prostacyclin: an inflammatory paradox Jeremiah Stitham, Charles Midgett, Kathleen A. Martin and John Hwa* Section of Cardiovascular Medicine, Department of Internal Medicine, Yale School of Medicine, Yale University, New Haven, CT, USA Edited by: Prostacyclin (PGI2) is a member of the prostaglandin family of bioactive lipids. Its best- Angel Lanas, University of Zaragoza, characterized role is in the cardiovascular system, where it is released by vascular endothelial cells, Spain serving as a potent vasodilator and inhibitor of platelet aggregation. In recent years, prostacyclin Reviewed by: Emer Smyth, University of (PGI2) has also been shown to promote differentiation and inhibit proliferation in vascular smooth Pennsylvania, USA muscle cells. In addition to these well-described homeostatic roles within the cardiovascular Steven W. Kerrigan, Royal College of system, prostacyclin (PGI2) also plays an important role as an inflammatory mediator. In this Surgeons in Ireland, Ireland review, we focus on the contribution of prostacyclin (PGI2) as both a pathophysiological mediator *Correspondence: and therapeutic agent in three major inflammatory-mediated disease processes, namely John Hwa, Section of Cardiovascular Medicine, Department of Internal rheumatoid arthritis, where it promotes disease progression (“pro-inflammatory”), along Medicine, Yale School of Medicine, with pulmonary vascular disease and atherosclerosis, where it inhibits disease progression Cardiovascular Research Center, 300 (“anti-inflammatory”). The emerging role of prostacyclin (PGI2) in this context provides new George Street, Room 759H, New opportunities for understanding the complex molecular basis for inflammatory-related diseases, Haven, CT 06511, USA. e-mail: [email protected] and insights into the development of current and future anti-inflammatory treatments. -

09 Non-Steroidal Anti-Inflammatory Drugs (Nsaids)
Part I Anaesthesia Refresher Course – 2017 09 University of Cape Town Non-steroidal anti-inflammatory drugs (NSAIDs) Dr Ernest Welch Private Practice Honorary lecturer- Wits University Non-steroidal anti-inflammatory drugs (NSAIDs) are a group of unrelated chemical compounds that have analgesic, anti-inflammatory and antipyretic effects. The similarity in therapeutic actions and side effects is due to common mechanisms of action and as a result they can be studied as a single class of drugs. The understanding of the NSAIDs, their effects and controversies is dependent on knowledge of the COX (cyclo-oxygenase) enzyme system. Mechanism of action Basic physiology of the COX pathway: 1. Fatty acid metabolism results in the production of prostaglandins (PG) via the COX pathway. 2. PGs mediate: inflammation, pain, pyrexia, cell mitosis and neuromuscular function. 3. All NSAIDs inhibit cyclo-oxygenase (COX). Formation of prostaglandin Arachidonic acid (AA) is a phospholipid fatty acid found in cell membranes that is released by a variety of stimuli particularly membrane damage. Cyclo-oxygenase (COX) and lipoxygenase (LOX) enzymes convert Arachidonic acid (AA) to lipid mediator’s PG and leukotrienes (also known as the eicosanoids) The two COX isoforms (COX-1 and COX-2) catalyse AA to PG and thromboxane (TxA) Initially COX converts AA to prostaglandin G2 (PGG2), and then converts PGG2 to prostaglandin H2 (PGH2) PGH2 is converted to 5 active forms of PG prostaglandin D2 (PGD2), prostaglandin E2 (PGE2) prostaglandin F2α (PGF2α) prostacyclin (PGI2) thromboxane A2 (TxA2) These 5 prostanoids act as secondary messengers mainly on G protein-coupled receptors. THE SYNTHESIS OF PROSTOGLANDINS USING CYCLO-OXYGENASE S. -

Acetylation by Aspirin (Prostaglandin Synthase/Prostacyclin/Thromboxane A2/Human Platelets/Acetylsalicylic Acid) JOHN W
Proc. Natl. Acad. Sci. USA Vol. 75, No. 10, pp. 5181-5184, October 1978 Medical Sciences Sensitivity of fatty acid cyclooxygenase from human aorta to acetylation by aspirin (prostaglandin synthase/prostacyclin/thromboxane A2/human platelets/acetylsalicylic acid) JOHN W. BURCH, NANCY LEWIS BAENZIGER, NANCY STANFORD, AND PHILIP W. MAJERUS* Division of Hematology-Oncology, Department of Internal Medicine, Washington University School of Medicine, St. Louis, Missouri 63110 Communicated by P. Roy Vagelos, July 28, 1978 ABSTRACT The rate of acetylation of fatty acid cyclooxy- from Nu Chek Prep, Elysian, MN. Nonidet P40 was purchased genase (prostaglandin synthase, EC 1.14.99.1) by [acetyl-3H- from Particle Data Laboratories, Elmhurst, IL. aspirin was measured in microsomes from human aortas and Indomethacin; coronary arteries and intact and disrupted human platelets. We bovine hemoglobin, type I; reduced glutathione; and epi- also measured the inhibition by aspirin of prostacyclin gener- nephrine bitartrate were purchased from Sigma Chemical Co., ation from exogenous arachidonic acid in shredded human St. Louis, MO. All other chemicals were reagent grade. aorta. Cyclooxygenase in human aorta and coronary artery Acetylation of Vascular Microsomes. Frozen aortas or microsomes is approximately 1/250th as sensitive to aspirin as coronary arteries were shredded with a hand-held enzyme in intact platelets, and 1/60th as sensitive to aspirin as vegetable enzyme measured in a platelet microsomal preparation. On the shredder or crushed with a hammer. Care was taken to keep basis of the in vitro data presented, we predict that small oral the tissues frozen (i.e., in contact with dry ice) during these doses of aspirin are sufficient to inhibit platelet prostaglandin procedures. -

Which COX-Inhibitor to Which Patient; an Analysis of Contemporary Evidence Including Pharmacology and Medicinal Chemistry
Institutionen för Kemi och Biomedicin Examensarbete Which COX-inhibitor to which patient; an analysis of contemporary evidence including pharmacology and medicinal chemistry Jakob Persson Huvudområde: Farmaci Nivå: Grundnivå Nr: X Which COX-inhibitor to which patient; an analysis of contemporary evidence including pharmacology and medicinal chemistry Jakob Persson Thesis in Pharmacy, 15 credits Bachelor of Science Program in Pharmacy, 180 credits Department of Chemistry and Biomedical Sciences Linnaeus University, Kalmar Supervisor Associate professor Ran Friedman Institutionen för kemi och biomedicinska vetenskaper Linnéuniversitetet SE-391 82 Kalmar Examinator Prof. Alf Månsson Institutionen för kemi och biomedicinska vetenskaper Linnéuniversitetet SE-391 82 Kalmar Abstract. NSAIDs are among the most used drugs in the world. It is estimated that 30 million people take NSAIDs daily world-wide, without including drugs sold over the counter. They are effective in alleviating pain and inflammation. Even though they are very common there does not appear to be any clear-cut guidelines to when which NSAID should be used. It has therefore been the purpose of this thesis to analyze if there is a need to differentiate between different NSAIDs according to contemporary evidence. Since the withdrawal of rofecoxib in 2004 there has been a general idea that coxibs as a group are cardiotoxic, recent evidence suggests that this holds true for all NSAIDs however. As such this work included 5 drugs, three common over the counter non-selective NSAIDs; naproxen, ibuprofen and diclofenac as well as the two coxibs currently on the Swedish market; celecoxib and etoricoxib. Pubmed and google scholar were searched for relevant studies on the subject.