Role of Arachidonic Acid and Its Metabolites in the Biological and Clinical Manifestations of Idiopathic Nephrotic Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Aryl Hydrocarbon Receptor: Structural Analysis and Activation Mechanisms
The Aryl Hydrocarbon Receptor: Structural Analysis and Activation Mechanisms This thesis is submitted in fulfilment of the requirements for the degree of Doctor of Philosophy in the School of Molecular and Biomedical Sciences (Biochemistry), The University of Adelaide, Australia Fiona Whelan, B.Sc. (Hons) 2009 2 Table of Contents THESIS SUMMARY................................................................................. 6 DECLARATION....................................................................................... 7 PUBLICATIONS ARISING FROM THIS THESIS.................................... 8 ACKNOWLEDGEMENTS...................................................................... 10 ABBREVIATIONS ................................................................................. 12 CHAPTER 1: INTRODUCTION ............................................................. 17 1.1 BHLH.PAS PROTEINS ............................................................................................17 1.1.1 General background..................................................................................17 1.1.2 bHLH.PAS Class I Proteins.........................................................................18 1.2 THE ARYL HYDROCARBON RECEPTOR......................................................................19 1.2.1 Domain Structure and Ligand Activation ..............................................19 1.2.2 AhR Expression and Developmental Activity .......................................21 1.2.3 Mouse AhR Knockout Phenotype ...........................................................23 -

PRODUCT INFORMATION Mefenamic Acid Item No
PRODUCT INFORMATION Mefenamic Acid Item No. 23650 CAS Registry No.: 61-68-7 OH Formal Name: 2-[(2,3-dimethylphenyl)amino]-benzoic acid O Synonyms: C.I. 473, CN 35355, NSC 94437 H C H NO MF: 15 15 2 N FW: 241.3 Purity: ≥98% Supplied as: A solid Storage: 4°C Stability: ≥1 year Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Mefenamic acid is supplied as a solid. A stock solution may be made by dissolving the mefenamic acid in the solvent of choice, which should be purged with an inert gas. Mefenamic acid is slightly soluble in DMSO and methanol. Description Mefenamic acid is a non-steroidal anti-inflammatory drug (NSAID) and a COX-2 inhibitor.1 It binds to COX-2 (Kd = 4 nM) and inhibits COX-2-dependent oxygenation of arachidonic acid (Item Nos. 90010 | 90010.1 | 10006607) in vitro (Ki = 10 µM). Mefenamic acid (30 mg/kg) reduces acetic acid-induced writhing in rats.2 It inhibits increases in skin thickness in a mouse model of delayed-type hypersensitivity induced by dinitrochlorobenzene (DNBC).2 Mefenamic acid also reduces the antibody response to sheep red blood cells in mice. References 1. Prusakiewicz, J.J., Duggan, K.C., Rouzer, C.A., et al. Differential sensitivity and mechanism of inhibition of COX-2 oxygenation of arachidonic acid and 2-arachidonoylglycerol by ibuprofen and mefenamic acid. Biochemistry 48(31), 7353-7355 (2009). 2. Roszkowski, A.P., Rooks, W.H., Tomolonis, A.J., et al. Anti-inflammatory and analgetic properties of d-2-(6’-methoxy-2’-naphthyl)-propionic acid (naproxen). -

AA Metabolism, 139 A2b1, 69, 70, 318–323 ABCB1 Gene, 176 Abciximab
Index A aIIbb3, 60, 62, 69, 70, 73, 74, 79, 80 AA. See Arachidonic acid (AA) AJW202, 293 AA metabolism, 139 Akt-1, 345 a2b1, 69, 70, 318–323 Akt-2, 345 ABCB1 gene, 176 ALX-0081, 296 Abciximab, 206–208, 497, 500, 501, 504–505, ALX-0681, 298 509, 513, 514, 525 Anagrelide, 227, 229 approval, 206 Ankle brachial indexes (ABIs), 549, 587, EPIC trial, 207 595–597 EPILOG study, 207 framingham risk score, 551 EPISTENT study, 207 screening, 550 platelet binding, 207 sensitivity, 551 thrombocytopenia, 206 specificity, 551 in unstable angina, 208 vascular risk, 550 ABIs. See Ankle brachial indexes (ABIs) Antagomirs, 439 Acetylsalicylic acid (aspirin), 137–158 Antibodies, 317 ACS. See Acute coronary syndromes (ACS) Anticoagulants, 525, 537 Acute coronary syndromes (ACS), 288 Anticoagulation, 533, 534 Acute ischemic stroke, 143, 149–150 Antioxidant, 233 Acute myocardial infarction, 142, 143, 149, Antiplatelet agents, 263, 553 153, 157 Antithrombotic Trialist’s ADAMTS-13, 93 Collaboration, 553 Adenosine diphosphate (ADP), 37, 88, 96, aspirin, 553 166, 473 clopidogrel, 553 Adenosine triphosphate (ATP), 37 dipyridamole, 553 Adhesion, 90, 112–118, 125 meta-analysis, 560 Adhesion molecules infiltration, 264 picotamide, 553 Adiponectin, 315 risk reduction, 553 ADP. See Adenosine diphosphate (ADP) ticlopidine, 553 ADP inhibitors (or P2Y12 blockers), 497–499, vascular events, 553 501–505, 507–509, 513, 514 Antiplatelet therapy, 472 ADP-receptor antagonists, 169 Apixaban, 535, 539 Adverse effects, 153–156 Aptamers, 299–301 Aegyptin, 327 Arachidonic acid (AA), 474 P. Gresele et al. (eds.), Antiplatelet Agents, Handbook of Experimental 607 Pharmacology 210, DOI 10.1007/978-3-642-29423-5, # Springer-Verlag Berlin Heidelberg 2012 608 Index ARC1779, 299–300 TXS signaling, 279 ARC15105, 300 Cardiovascular death, 248 AR-C69931MX, 456 Carotid endarterectomy (ACE) inhibition, Aspirin, 474, 496, 498–499, 501–502, 143, 156 504–507, 511–514, 520, 522, Carotid stenosis, 523 527, 531, 533–537, 539, CCBs. -

Open Thesis Master Document V5.0.Pdf
The Pennsylvania State University The Graduate School Department of Veterinary and Biomedical Science IDENTIFICATION OF ENDOGENOUS MODULATORS FOR THE ARYL HYDROCARBON RECEPTOR A Thesis in Genetics by Christopher R. Chiaro © 2007 Christopher R. Chiaro Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December, 2007 The thesis of Christopher R. Chiaro was reviewed and approved* by the following: Gary H. Perdew John T. and Paige S. Smith Professor in Agricultural Sciences Thesis Advisor Chair of Committee C. Channa Reddy Distinguished Professor of Veterinary Science A. Daniel Jones Senior Scientist Department of Chemistry John P. Vanden Heuvel Professor of Veterinary Science Richard Ordway Associate Professor of Biology Chair of Genetics Graduate Program *Signatures are on file in the Graduate School iii ABSTRACT The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor capable of being regulated by a structurally diverse array of chemicals ranging from environmental carcinogens to dietary metabolites. A member of the basic helix-loop- helix/ Per-Arnt-Sim (bHLH-PAS) super-family of DNA binding regulatory proteins, the AhR is an important developmental regulator that can be detected in nearly all mammalian tissues. Prior to ligand activation, the AhR resides in the cytosol as part of an inactive oligomeric protein complex comprised of the AhR ligand-binding subunit, a dimer of the 90 kDa heat shock protein, and a single molecule each of the immunophilin like X-associated protein 2 (XAP2) and p23 proteins. Functioning as chemosensor, the AhR responds to both endobiotic and xenobiotic derived chemical ligands by ultimately directing the expression of metabolically important target genes. -

N-Acyl-Dopamines: Novel Synthetic CB1 Cannabinoid-Receptor Ligands
Biochem. J. (2000) 351, 817–824 (Printed in Great Britain) 817 N-acyl-dopamines: novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo Tiziana BISOGNO*, Dominique MELCK*, Mikhail Yu. BOBROV†, Natalia M. GRETSKAYA†, Vladimir V. BEZUGLOV†, Luciano DE PETROCELLIS‡ and Vincenzo DI MARZO*1 *Istituto per la Chimica di Molecole di Interesse Biologico, C.N.R., Via Toiano 6, 80072 Arco Felice, Napoli, Italy, †Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, R. A. S., 16/10 Miklukho-Maklaya Str., 117871 Moscow GSP7, Russia, and ‡Istituto di Cibernetica, C.N.R., Via Toiano 6, 80072 Arco Felice, Napoli, Italy We reported previously that synthetic amides of polyunsaturated selectivity for the anandamide transporter over FAAH. AA-DA fatty acids with bioactive amines can result in substances that (0.1–10 µM) did not displace D1 and D2 dopamine-receptor interact with proteins of the endogenous cannabinoid system high-affinity ligands from rat brain membranes, thus suggesting (ECS). Here we synthesized a series of N-acyl-dopamines that this compound has little affinity for these receptors. AA-DA (NADAs) and studied their effects on the anandamide membrane was more potent and efficacious than anandamide as a CB" transporter, the anandamide amidohydrolase (fatty acid amide agonist, as assessed by measuring the stimulatory effect on intra- hydrolase, FAAH) and the two cannabinoid receptor subtypes, cellular Ca#+ mobilization in undifferentiated N18TG2 neuro- CB" and CB#. NADAs competitively inhibited FAAH from blastoma cells. This effect of AA-DA was counteracted by the l µ N18TG2 cells (IC&! 19–100 M), as well as the binding of the CB" antagonist SR141716A. -

Types of Gene Effects Governing the Inheritance of Oleic and Linoleic Acids in Peanut (Arachis Hypogaea L.)
African Journal of Biotechnology Vol. 11(67), pp. 13147-13152, 21 August, 2012 Available online at http://www.academicjournals.org/AJB DOI:10.5897/AJB12.1498 ISSN 1684-5315 ©2012 Academic Journals Full Length Research Paper Types of gene effects governing the inheritance of oleic and linoleic acids in peanut (Arachis hypogaea L.) Nattawut Singkham1, Sanun Jogloy1*, Bhalang Suriharn1, Thawan Kesmala1, Prasan Swatsitang2, Prasit Jaisil1, Naveen Puppala3 and Aran Patanothai1 1Department of Plant Science and Agricultural Resources, Faculty of Agriculture, Khon Kaen University, Khon Kaen, 40002, Thailand. 2Department of Biochemistry, Faculty of Science, Khon Kaen University, Khon Kaen, 40002, Thailand. 3Agricultural Science Center at Clovis, New Mexico State University, Clovis, New Mexico, 88101, USA. Accepted 3 August, 2012 Oleic and linoleic acids are major fatty acids in peanut determining the quality and shelf-life of peanut products. A better understanding on the inheritance of these characters is an important for high-oleic breeding programs. The objective of this research was to determine the gene actions for oleic acid, linoleic acid, the ratio of oleic to linoleic acids (O/L ratio) and percentage oil (% oil) in peanut. Georgia- 02C, SunOleic 97R (high-oleic genotypes) and KKU 1 (low-oleic genotypes) were used as parents to generate P1, P2, F2, F3, BC11S and BC12S. The entries were planted in a randomized complete block design with four replications in the rainy season (2008) and the dry season (2008/2009). Gas liquid chromatography (GLC) was used to analyze fatty acid compositions. The data were used in generation means analysis to understand gene effects. The differences in season, generation and generation season interactions were significant for oleic acid in the crosses Georgia-02C KKU 1 and SunOleic 97R KKU 1. -

Epigenetic Regulations of Ahr in the Aspect of Immunomodulation
International Journal of Molecular Sciences Review Epigenetic Regulations of AhR in the Aspect of Immunomodulation Anna Wajda 1,* , Joanna Łapczuk-Roma ´nska 2 and Agnieszka Paradowska-Gorycka 1 1 Department of Molecular Biology, National Institute of Geriatrics, Rheumatology and Rehabilitation, 02-637 Warsaw, Poland; [email protected] 2 Department of Experimental and Clinical Pharmacology, Pomeranian Medical University, 70-111 Szczecin, Poland; [email protected] * Correspondence: [email protected] Received: 31 July 2020; Accepted: 28 August 2020; Published: 3 September 2020 Abstract: Environmental factors contribute to autoimmune disease manifestation, and as regarded today, AhR has become an important factor in studies of immunomodulation. Besides immunological aspects, AhR also plays a role in pharmacological, toxicological and many other physiological processes such as adaptive metabolism. In recent years, epigenetic mechanisms have provided new insight into gene regulation and reveal a new contribution to autoimmune disease pathogenesis. DNA methylation, histone modifications, chromatin alterations, microRNA and consequently non-genetic changes in phenotypes connect with environmental factors. Increasing data reveals AhR cross-roads with the most significant in immunology pathways. Although study on epigenetic modulations in autoimmune diseases is still not well understood, therefore future research will help us understand their pathophysiology and help to find new therapeutic strategies. Present literature review -

Environmental Pollution 275 (2021) 116665
Environmental Pollution 275 (2021) 116665 Contents lists available at ScienceDirect Environmental Pollution journal homepage: www.elsevier.com/locate/envpol Metabolomics reveals the reproductive abnormality in female zebrafish exposed to environmentally relevant levels of climbazole* Ting Zou a, 1, Yan-Qiu Liang b, 1, Xiaoliang Liao a, Xiao-Fan Chen a, Tao Wang c, * Yuanyuan Song c, Zhi-Cheng Lin a, Zenghua Qi a, Zhi-Feng Chen a, d, , Zongwei Cai a, c a Guangdong Key Laboratory of Environmental Catalysis and Health Risk Control, School of Environmental Science and Engineering, Institute of Environmental Health and Pollution Control, Guangdong University of Technology, Guangzhou, 510006, China b Faculty of Chemistry and Environmental Science, Guangdong Ocean University, Zhanjiang, 524088, China c State Key Laboratory of Environmental and Biological Analysis, Department of Chemistry, Hong Kong Baptist University, Hong Kong Special Administrative Region, China d Guangdong Provincial Key Laboratory of Chemical Pollution and Environmental Safety, South China Normal University, Guangzhou, 510006, China article info abstract Article history: Climbazole (CBZ) ubiquitously detected in the aquatic environment may disrupt fish reproductive Received 15 November 2020 function. Thus far, the previous study has focused on its transcriptional impact of steroidogenesis-related Received in revised form genes on zebrafish, but the underlying toxic mechanism still needs further investigation at the metabolic 10 January 2021 level. In this study, adult zebrafish were chronically exposed to CBZ at concentrations of 0.1 (corre- Accepted 2 February 2021 sponding to the real concentration in surface water), 10, and 1000 mg/L and evaluated for reproductive Available online 5 February 2021 function by egg production, with subsequent ovarian tissue samples taken for histology, metabolomics, and other biochemical analysis. -

Use of Gamma-Linolenic Acid and Related Compounds for the Manufacture of a Medicament for the Treatment of Endometriosis
~" ' MM II II II II I II Ml Ml Ml I II I II J European Patent Office ooo Ats*% n i © Publication number: 0 222 483 B1 Office_„. europeen des brevets © EUROPEAN PATENT SPECIFICATION © Date of publication of patent specification: 18.03.92 © Int. CI.5: A61 K 31/20, A61 K 31/1 6, A61K 31/23 © Application number: 86307533.9 @ Date of filing: 01.10.86 Use of gamma-linolenic acid and related compounds for the manufacture of a medicament for the treatment of endometriosis. © Priority: 02.10.85 GB 8524276 © Proprietor: EFAMOL HOLDINGS PLC Efamol House Woodbridge Meadows @ Date of publication of application: Guildford Surrey GU1 1BA(GB) 20.05.87 Bulletin 87/21 @ Inventor: Horrobin, David Frederick © Publication of the grant of the patent: c/o Efamol Ltd, Efamol House Woodbridge 18.03.92 Bulletin 92/12 Meadows Guildford, Surrey, GU1 1BA(GB) © Designated Contracting States: Inventor: Casper, Robert AT BE CH DE ES FR GB GR IT LI LU NL SE University Hospital 339 Windermere Road London Ontario N6A 5AS(CA) © References cited: EP-A- 0 003 407 EP-A- 0 115 419 © Representative: Miller, Joseph EP-A- 0 132 089 J. MILLER & CO. Lincoln House 296-302 High EP-A- 0 181 689 Holborn London WC1V 7JH(GB) J. GYNECOL. OBSTET. BIOL. REPROD. vol. 10, no. 5, 1981, pages 465-471 Masson, Paris, FR PH. CALLGARIS et al.: "Endometriose de la paroi abdominale" 00 00 CLINICAL OBSTETRICS AND GYNECOLOGY, 00 vol. 23, no. 3, Sept. 1980, pages 895-900 J.C. WEED: "Prostaglandins as related to en- CM dometriosis" CM CM Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

THE L5178Y SUBLINES: a SUMMARY of 40 YEAR STUDIES in WARSAW Irena Szumiel
ISSN 1425-7351 PL0601824 RAPORTY IChTJ. SERIA B nr 2/2005 THE L5178Y SUBLINES: A SUMMARY OF 40 YEAR STUDIES IN WARSAW Irena Szumiel INSTYTUT CHEMII I TECHNIKI JĄDROWEJ INSTITUTE OF NUCLEAR CHEMISTRY AND TECHNOLOGY RAPORTY IChTJ. SERIA B nr 2/2005 THE L5178Y SUBLINES: A SUMMARY OF 40 YEAR STUDIES IN WARSAW Irena Szumiel Warszawa 2005 AUTHOR Irena Szumiel Department of Radiobiology and Health Protection, Institute of Nuclear Chemistry and Technology This report is an extended version of the paper: " 'The L5178Y sublines: a look back from 40 years. 1.General characteristics. 2. Response to ionising radiation", published in International Journal of Radiation Biology, vol. 81 (2005) by kind permission of Taylor & Francis (http://www. tandf.co.uk) ADDRESS OF THE EDITORIAL OFFICE Institute of Nuclear Chemistry and Technology Dorodna 16, 03-195 Warszawa, POLAND phone: (+4822) 811 06 56, fax: (+4822) 81115 32, e-mail: [email protected] Papers are published in the form as received from the Authors The L5178Y sublines: a summary of 40 year studies in Warsaw The purpose of this report has been to review and summarise the results of 40 year studies concerning the general characteristics and response to UVC radiation, hydrogen peroxide and ionising radiation of the pair of L5178Y (LY) sublines, LY-R and LY-S, that differ in sen- sitivity to various DNA damaging agents. Comparison of karyotypes shows a number of differences in the banding patterns. Differences are found in ion transport and the ganglioside pattern of the plasma membranes, as well as in the content and turnover rate of poly(ADP-ribose) polymers. -

Lipoxin A4: a Novel Anti-Inflammatory Molecule? Thorax: First Published As 10.1136/Thx.50.2.111 on 1 February 1995
Thorax 1995;50:111-112 illl Lipoxin A4: a novel anti-inflammatory molecule? Thorax: first published as 10.1136/thx.50.2.111 on 1 February 1995. Downloaded from Arachidonic acid is metabolised by the cyclooxygenase contractions.22 These studies support the view that LXA4 pathway to the prostaglandins and thromboxane A2 or via may act as a partial agonist at the same or similar site as one of the lipoxygenase pathways.' Three major lip- the sulphidopeptide leukotrienes. oxygenase pathways have been identified in mammalian The fact that 1 5-lipoxygenase is abundant in lung tissue tissue - namely, the 5-, 12-, and 15-lipoxygenases.2A The and that LXA4 has been recovered in the bronchoalveolar 5-lipoxygenase pathway metabolises arachidonic acid lavage fluid ofpatients with asthma and other lung diseases through two intermediates, 5-hydroperoxyeicosatetranoic suggests that LXA4 may be a potential mediator or mod- acid (5-HPETE) and leukotriene A4 (LTA4), to LTB4 and ulator of inflammation in the lung. In a recent study eight the sulphidopeptide leukotrienes LTC4, LTD4, and LTE4.5 subjects underwent inhalation challenge with LXA4,23 but The sulphidopeptide leukotrienes are potent spasmogens6 no effect was seen on specific conductance, rate of airflow for non-vascular smooth muscle and may play a part in at 25% vital capacity (V25), blood pressure, pulse, or the pathogenesis of bronchial asthma.7`10 asthmatic symptoms. There was, however, a significant The interactions between 5-lipoxygenase and 15-lip- shift of the specific conductance and V25 dose-response oxygenase on arachidonic acid metabolism have recently curve to the right after inhalation challenge with LTC4 been studied and a new series of biologically active me- combined with LXA4 compared with that after inhalation tabolites described." 12 These molecules have been termed challenge with LTC4 alone. -
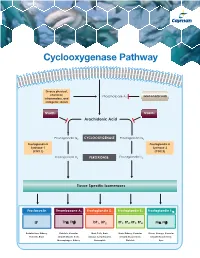
Cyclooxygenase Pathway
Cyclooxygenase Pathway Diverse physical, chemical, Phospholipase A Glucocorticoids inflammatory, and 2 mitogenic stimuli NSAIDs NSAIDs Arachidonic Acid Prostaglandin G2 CYCLOOXYGENASE Prostaglandin G2 Prostaglandin H Prostaglandin H Synthase-1 Synthase-2 (COX 1) (COX 2) Prostaglandin H2 PEROXIDASE Prostaglandin H2 Tissue Specific Isomerases Prostacyclin Thromboxane A2 Prostaglandin D2 Prostaglandin E2 Prostaglandin F2α IP TPα, TPβ DP1, DP2 EP1, EP2, EP3, EP4 FPα, FPβ Endothelium, Kidney, Platelets, Vascular Mast Cells, Brain, Brain, Kidney, Vascular Uterus, Airways, Vascular Platelets, Brain Smooth Muscle Cells, Airways, Lymphocytes, Smooth Muscle Cells, Smooth Muscle Cells, Macrophages, Kidney Eosinophils Platelets Eyes Prostacyclin Item No. Product Features Prostacyclin (Prostaglandin I2; PGI2) is formed from arachidonic acid primarily in the vascular endothelium and renal cortex by sequential 515211 6-keto • Sample Types: Culture Medium | Plasma Prostaglandin • Measure 6-keto PGF levels down to 6 pg/ml activities of COX and prostacyclin synthase. PGI2 is non-enzymatically 1α F ELISA Kit • Incubation : 18 hours | Development: 90-120 minutes | hydrated to 6-keto PGF1α (t½ = 2-3 minutes), and then quickly converted 1α Read: Colorimetric at 405-420 nm to the major metabolite, 2,3-dinor-6-keto PGF1α (t½= 30 minutes). Prostacyclin was once thought to be a circulating hormone that regulated • Assay 24 samples in triplicate or 36 samples in duplicate platelet-vasculature interactions, but the rate of secretion into circulation • NOTE: A portion of urinary 6-keto PGF1α is of renal origin coupled with the short half-life indicate that prostacyclin functions • NOTE : It has been found that normal plasma levels of 6-keto PGF may be low locally.