Prostaglandin E Receptor 3 Signaling Is Induced in Placentas With
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Suppression of Prostate Tumor Cell Growth by Stromal Cell Prostaglandin D Synthase–Derived Products
Research Article Suppression of Prostate Tumor Cell Growth by Stromal Cell Prostaglandin D Synthase–Derived Products Jeri Kim,1 Peiying Yang,2 Milind Suraokar,3 Anita L. Sabichi,3 Norma D. Llansa,3 Gabriela Mendoza,3 Vemparalla Subbarayan,3 Christopher J. Logothetis,1 Robert A. Newman,2 Scott M. Lippman,3 and David G. Menter3 Departments of 1Genitourinary Medical Oncology, 2Experimental Therapeutics, and 3Clinical Cancer Prevention, The University of Texas M.D. Anderson Cancer Center, Houston, Texas Abstract seminal fluid (10). Once PGD2 is made, it forms derivative Stromal-epithelial interactions and the bioactive molecules compounds, most of which can transactivate the peroxisome g g produced by these interactions maintain tissue homeostasis proliferator–activated receptor (PPAR ). One PGD2 derivative, 15-deoxy-D12,14-prostaglandin J (15-d-PGJ ), can slow the growth and influence carcinogenesis. Bioactive prostaglandins pro- 2 2 duced by prostaglandin synthases and secreted by the prostate and induce the partial differentiation of selected cancer cells (12). D12,14 into seminal plasma are thought to support reproduction, but Another PGD2 derivative, 15-deoxy- -PGD2 (15-d-PGD2), has g their endogenous effects on cancer formation remain unre- also been shown to stimulate PPAR transactivation in RAW 264.7 solved. No studies to date have examined prostaglandin cell macrophage cultures as effectively as 15-d-PGJ2 (13). L-PGDS enzyme production or prostaglandin metabolism in normal also binds tritiated testosterone and may play a role in androgen prostate stromal cells. Our results show that lipocalin-type transport (14). In castrated rats, testosterone proprionate induces prostaglandin D synthase (L-PGDS) and prostaglandin D L-PGDS synthesis in the epididymis (15). -

Outpatient Acne Care Guideline
Outpatient Acne Care Guideline Severity Mild Moderate Severe < 20 comedones or < 20-100 comedones or 15-50 > 5 cysts, >100 comedones, or inflammatory lesions inflammatory lesions >50 inflammatory lesions Initial Treatment Initial Treatment Initial Treatment Benzoyl Peroxide (BP) or Topical Combination Therapy Combination Therapy Topical Retinoid Retinoid + BP Oral antibiotic or OR + (Retinoid + Antibiotic) + BP Topical retinoid Topical Combination Therapy or + BP + Antibiotic Retinoid + (BP + Antibiotic) or OR BP Retinoid + BP Oral antibiotic + topical retinoid + +/- or BP Topical antibiotic Retinoid + Antibiotic + BP or Topical Dapsone IF Inadequate Response IF Inadequate Response IF Inadequate Consider dermatology Response referral Change topical retinoid Consider changing oral concentrations, type and/or antibiotic formulation AND or Add BP or retinoid, if not already Change topiocal combination Consider isotretinoin prescribed therapy Consider hormone therapy or and/or (females) Change topical retinoid Add or change oral antibiotic concentrations, type and/or or formulation Consider isotretinoin Additional Considerations or Consider hormone therapy (females) Change topical comination Previous treatment/history Side effects therapy Costs Psychosocial impact Vehicle selection Active scarring Ease of use Regimen complexity Approved Evidence Based Medicine Committee 1-18-17 Reassess the appropriateness of Care Guidelines as condition changes. This guideline is a tool to aid clinical decision making. It is not a standard of care. The physician should deviate from the guideline when clinical judgment so indicates. GOAL: Pediatricians should initiate treatment for cases of “Mild” to “Severe” acne (see algorithms attached). Pediatricians should also counsel patients in order to maximize adherence to acne treatment regimens: 1. Realistic expectations. Patients should be counseled that topical therapies typically take up to 6-8 weeks to start seeing results. -

Structure-Based Discovery of Mpges-1 Inhibitors Suitable For
www.nature.com/scientificreports OPEN Structure-based discovery of mPGES-1 inhibitors suitable for preclinical testing in wild-type Received: 4 January 2018 Accepted: 9 March 2018 mice as a new generation of anti- Published: xx xx xxxx infammatory drugs Kai Ding1,2,3, Ziyuan Zhou1,2, Shurong Hou2, Yaxia Yuan1,2,4, Shuo Zhou1,2, Xirong Zheng2, Jianzhong Chen1,2, Charles Loftin2, Fang Zheng1,2 & Chang-Guo Zhan1,2,4 Human mPGES-1 is recognized as a promising target for next generation of anti-infammatory drugs without the side efects of currently available anti-infammatory drugs, and various inhibitors have been reported in the literature. However, none of the reported potent inhibitors of human mPGES-1 has shown to be also a potent inhibitor of mouse or rat mPGES-1, which prevents using the well-established mouse/rat models of infammation-related diseases for preclinical studies. Hence, despite of extensive eforts to design and discover various human mPGES-1 inhibitors, the promise of mPGES-1 as a target for the next generation of anti-infammatory drugs has never been demonstrated in any wild-type mouse/rat model using an mPGES-1 inhibitor. Here we report discovery of a novel type of selective mPGES-1 inhibitors potent for both human and mouse mPGES-1 enzymes through structure-based rational design. Based on in vivo studies using wild-type mice, the lead compound is indeed non-toxic, orally bioavailable, and more potent in decreasing the PGE2 (an infammatory marker) levels compared to the currently available drug celecoxib. This is the frst demonstration in wild-type mice that mPGES-1 is truly a promising target for the next generation of anti-infammatory drugs. -

AA Metabolism, 139 A2b1, 69, 70, 318–323 ABCB1 Gene, 176 Abciximab
Index A aIIbb3, 60, 62, 69, 70, 73, 74, 79, 80 AA. See Arachidonic acid (AA) AJW202, 293 AA metabolism, 139 Akt-1, 345 a2b1, 69, 70, 318–323 Akt-2, 345 ABCB1 gene, 176 ALX-0081, 296 Abciximab, 206–208, 497, 500, 501, 504–505, ALX-0681, 298 509, 513, 514, 525 Anagrelide, 227, 229 approval, 206 Ankle brachial indexes (ABIs), 549, 587, EPIC trial, 207 595–597 EPILOG study, 207 framingham risk score, 551 EPISTENT study, 207 screening, 550 platelet binding, 207 sensitivity, 551 thrombocytopenia, 206 specificity, 551 in unstable angina, 208 vascular risk, 550 ABIs. See Ankle brachial indexes (ABIs) Antagomirs, 439 Acetylsalicylic acid (aspirin), 137–158 Antibodies, 317 ACS. See Acute coronary syndromes (ACS) Anticoagulants, 525, 537 Acute coronary syndromes (ACS), 288 Anticoagulation, 533, 534 Acute ischemic stroke, 143, 149–150 Antioxidant, 233 Acute myocardial infarction, 142, 143, 149, Antiplatelet agents, 263, 553 153, 157 Antithrombotic Trialist’s ADAMTS-13, 93 Collaboration, 553 Adenosine diphosphate (ADP), 37, 88, 96, aspirin, 553 166, 473 clopidogrel, 553 Adenosine triphosphate (ATP), 37 dipyridamole, 553 Adhesion, 90, 112–118, 125 meta-analysis, 560 Adhesion molecules infiltration, 264 picotamide, 553 Adiponectin, 315 risk reduction, 553 ADP. See Adenosine diphosphate (ADP) ticlopidine, 553 ADP inhibitors (or P2Y12 blockers), 497–499, vascular events, 553 501–505, 507–509, 513, 514 Antiplatelet therapy, 472 ADP-receptor antagonists, 169 Apixaban, 535, 539 Adverse effects, 153–156 Aptamers, 299–301 Aegyptin, 327 Arachidonic acid (AA), 474 P. Gresele et al. (eds.), Antiplatelet Agents, Handbook of Experimental 607 Pharmacology 210, DOI 10.1007/978-3-642-29423-5, # Springer-Verlag Berlin Heidelberg 2012 608 Index ARC1779, 299–300 TXS signaling, 279 ARC15105, 300 Cardiovascular death, 248 AR-C69931MX, 456 Carotid endarterectomy (ACE) inhibition, Aspirin, 474, 496, 498–499, 501–502, 143, 156 504–507, 511–514, 520, 522, Carotid stenosis, 523 527, 531, 533–537, 539, CCBs. -

Antiluteogenic Effects of Serial Prostaglandin F2α Administration in Mares
ANTILUTEOGENIC EFFECTS OF SERIAL PROSTAGLANDIN F2α ADMINISTRATION IN MARES Thesis Presented in partial fulfillment of the requirements for the Master of Science in the Graduate School of The Ohio State University Elizabeth Ann Coffman Graduate ProGram in Comparative and Veterinary Medicine The Ohio State University 2013 Thesis committee: Carlos R.F. Pinto PhD, MV, DACT; Dissertation Advisor Marco A. Coutinho da Silva PhD, MV, DACT; Academic Advisor Christopher Premanandan PhD, DVM, DACVP Copyright by Elizabeth Ann Coffman 2013 Abstract For breedinG manaGement and estrus synchronization, prostaGlandin F2α (PGF) is one of the most commonly utilized hormones to pharmacologically manipulate the equine estrous cycle. There is a general supposition a sinGle dose of PGF does not consistently induce luteolysis in the equine corpus luteum (CL) until at least five to six days after ovulation. This leads to the erroneous assumption that the early CL (before day five after ovulation) is refractory to the luteolytic effects of PGF. An experiment was desiGned to test the hypotheses that serial administration of PGF in early diestrus would induce a return to estrus similar to mares treated with a sinGle injection in mid diestrus, and fertility of the induced estrus for the two treatment groups would not differ. The specific objectives of the study were to evaluate the effects of early diestrus treatment by: 1) assessing the luteal function as reflected by hormone profile for concentration of plasma progesterone; 2) determininG the duration of interovulatory and treatment to ovulation intervals; 3) comparing of the number of pregnant mares at 14 days post- ovulation. The study consisted of a balanced crossover desiGn in which reproductively normal Quarter horse mares (n=10) were exposed to two treatments ii on consecutive reproductive cycles. -

Role of Arachidonic Acid and Its Metabolites in the Biological and Clinical Manifestations of Idiopathic Nephrotic Syndrome
International Journal of Molecular Sciences Review Role of Arachidonic Acid and Its Metabolites in the Biological and Clinical Manifestations of Idiopathic Nephrotic Syndrome Stefano Turolo 1,* , Alberto Edefonti 1 , Alessandra Mazzocchi 2, Marie Louise Syren 2, William Morello 1, Carlo Agostoni 2,3 and Giovanni Montini 1,2 1 Fondazione IRCCS Ca’ Granda-Ospedale Maggiore Policlinico, Pediatric Nephrology, Dialysis and Transplant Unit, Via della Commenda 9, 20122 Milan, Italy; [email protected] (A.E.); [email protected] (W.M.); [email protected] (G.M.) 2 Department of Clinical Sciences and Community Health, University of Milan, 20122 Milan, Italy; [email protected] (A.M.); [email protected] (M.L.S.); [email protected] (C.A.) 3 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Pediatric Intermediate Care Unit, 20122 Milan, Italy * Correspondence: [email protected] Abstract: Studies concerning the role of arachidonic acid (AA) and its metabolites in kidney disease are scarce, and this applies in particular to idiopathic nephrotic syndrome (INS). INS is one of the most frequent glomerular diseases in childhood; it is characterized by T-lymphocyte dysfunction, alterations of pro- and anti-coagulant factor levels, and increased platelet count and aggregation, leading to thrombophilia. AA and its metabolites are involved in several biological processes. Herein, Citation: Turolo, S.; Edefonti, A.; we describe the main fields where they may play a significant role, particularly as it pertains to their Mazzocchi, A.; Syren, M.L.; effects on the kidney and the mechanisms underlying INS. AA and its metabolites influence cell Morello, W.; Agostoni, C.; Montini, G. -
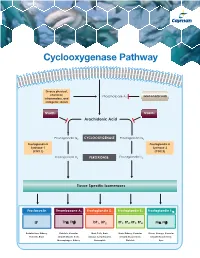
Cyclooxygenase Pathway
Cyclooxygenase Pathway Diverse physical, chemical, Phospholipase A Glucocorticoids inflammatory, and 2 mitogenic stimuli NSAIDs NSAIDs Arachidonic Acid Prostaglandin G2 CYCLOOXYGENASE Prostaglandin G2 Prostaglandin H Prostaglandin H Synthase-1 Synthase-2 (COX 1) (COX 2) Prostaglandin H2 PEROXIDASE Prostaglandin H2 Tissue Specific Isomerases Prostacyclin Thromboxane A2 Prostaglandin D2 Prostaglandin E2 Prostaglandin F2α IP TPα, TPβ DP1, DP2 EP1, EP2, EP3, EP4 FPα, FPβ Endothelium, Kidney, Platelets, Vascular Mast Cells, Brain, Brain, Kidney, Vascular Uterus, Airways, Vascular Platelets, Brain Smooth Muscle Cells, Airways, Lymphocytes, Smooth Muscle Cells, Smooth Muscle Cells, Macrophages, Kidney Eosinophils Platelets Eyes Prostacyclin Item No. Product Features Prostacyclin (Prostaglandin I2; PGI2) is formed from arachidonic acid primarily in the vascular endothelium and renal cortex by sequential 515211 6-keto • Sample Types: Culture Medium | Plasma Prostaglandin • Measure 6-keto PGF levels down to 6 pg/ml activities of COX and prostacyclin synthase. PGI2 is non-enzymatically 1α F ELISA Kit • Incubation : 18 hours | Development: 90-120 minutes | hydrated to 6-keto PGF1α (t½ = 2-3 minutes), and then quickly converted 1α Read: Colorimetric at 405-420 nm to the major metabolite, 2,3-dinor-6-keto PGF1α (t½= 30 minutes). Prostacyclin was once thought to be a circulating hormone that regulated • Assay 24 samples in triplicate or 36 samples in duplicate platelet-vasculature interactions, but the rate of secretion into circulation • NOTE: A portion of urinary 6-keto PGF1α is of renal origin coupled with the short half-life indicate that prostacyclin functions • NOTE : It has been found that normal plasma levels of 6-keto PGF may be low locally. -

Effect of Prostanoids on Human Platelet Function: an Overview
International Journal of Molecular Sciences Review Effect of Prostanoids on Human Platelet Function: An Overview Steffen Braune, Jan-Heiner Küpper and Friedrich Jung * Institute of Biotechnology, Molecular Cell Biology, Brandenburg University of Technology, 01968 Senftenberg, Germany; steff[email protected] (S.B.); [email protected] (J.-H.K.) * Correspondence: [email protected] Received: 23 October 2020; Accepted: 23 November 2020; Published: 27 November 2020 Abstract: Prostanoids are bioactive lipid mediators and take part in many physiological and pathophysiological processes in practically every organ, tissue and cell, including the vascular, renal, gastrointestinal and reproductive systems. In this review, we focus on their influence on platelets, which are key elements in thrombosis and hemostasis. The function of platelets is influenced by mediators in the blood and the vascular wall. Activated platelets aggregate and release bioactive substances, thereby activating further neighbored platelets, which finally can lead to the formation of thrombi. Prostanoids regulate the function of blood platelets by both activating or inhibiting and so are involved in hemostasis. Each prostanoid has a unique activity profile and, thus, a specific profile of action. This article reviews the effects of the following prostanoids: prostaglandin-D2 (PGD2), prostaglandin-E1, -E2 and E3 (PGE1, PGE2, PGE3), prostaglandin F2α (PGF2α), prostacyclin (PGI2) and thromboxane-A2 (TXA2) on platelet activation and aggregation via their respective receptors. Keywords: prostacyclin; thromboxane; prostaglandin; platelets 1. Introduction Hemostasis is a complex process that requires the interplay of multiple physiological pathways. Cellular and molecular mechanisms interact to stop bleedings of injured blood vessels or to seal denuded sub-endothelium with localized clot formation (Figure1). -

Therapeutic Drug Class
BUREAU FOR MEDICAL SERVICES WEST VIRGINIA MEDICAID EFFECTIVE PREFERRED DRUG LIST WITH PRIOR AUTHORIZATION CRITERIA 04/01/11 This is not an all-inclusive list of available covered drugs and includes only Version 2011.9 managed categories. Refer to cover page for complete list of rules governing this PDL. • Prior authorization for a non-preferred agent in any category will be given only if there has been a trial of the preferred brand/generic equivalent or preferred formulation of the active ingredient, at a therapeutic dose, that resulted in a partial response with a documented intolerance. • Prior authorization of a non-preferred isomer, pro-drug, or metabolite will be considered with a trial of a preferred parent drug of the same chemical entity, at a therapeutic dose, that resulted in a partial response with documented intolerance or a previous trial and therapy failure, at a therapeutic dose, with a preferred drug of a different chemical entity indicated to treat the submitted diagnosis. (The required trial may be overridden when documented evidence is provided that the use of these preferred agent(s) would be medically contraindicated.) • Unless otherwise specified, the listing of a particular brand or generic name includes all legend forms of that drug. OTC drugs are not covered unless specified. • PA criteria for non-preferred agents apply in addition to general Drug Utilization Review policy that is in effect for the entire pharmacy program, including, but not limited to, appropriate dosing, duplication of therapy, etc. • The use of pharmaceutical samples will not be considered when evaluating the members’ medical condition or prior prescription history for drugs that require prior authorization. -

Medical Management of First-Trimester Induced Abortion and Miscarriage
PACE REVIEW Medical management of first-trimester induced abortion and miscarriage Shamim Amis Jonathon Evans-Jones MRCOG FKCOG urgical evacuation is the mainstay of treatment in the effects of bleeding per vaginum, diarrhoea and vomiting. In UK for first-trimester termination of pregnancy and a randomised trial, where 1 mg versus 0.5 mg of gemeprost Smiscarriage and, although a niinor procedure, it has an was compared, the complete abortion rate was similar for associated considerable morbidity and mortality.' Induced the two groups (98-100%), although the incidence of abortion in the UK is now a safe procedure but on a global adverse effects was significantly lower in the latter group.'O scale continues to be a major cause of maternal mortality.2 Misoprosto1 is a synthetic analogue of prostaglandin E, and Medical management would provide a safe and effective causes increased uterine contractility with a low incidence of alternative. Many women would prefer to be given the other unwanted effects." The main advantages over choice and avoid the risks associated with anaesthesia and gemeprost are that it does not require refngeration, is cheaper surgery.l Recent studies have confirmed high acceptability and can be administered orally or vaginally. One gemeprost rates, showing that 8496% of women would choose pessary costs &22, whereas the equivalent dose of misoprostol medical treatment for a subsequent abortion." is just over The uterotonic properties are enkanced if women are pretreated with rmfepristone, reflecting the effect BACKGROUND of antiprogesterones in increasing sensitivity to prostaglandins. The drugs used for medically induced abortion in the UK When misoprostol is used in combination with mifepristone, include an antiprogesterone,mifepristone, and several prost- the vaginal route has been shown to be superior to the onl aglandin analogues, including gemeprost and misoprostol. -

2374 Supplementary Drugs and Other Substances
2374 Supplementary Drugs and Other Substances 5. Burdock GA. Review of the biological properties and toxicity of Pharmacokinetics reduced to the endoperoxide prostaglandin H2 (PGH2). Prostag- bee propolis (propolis). Food Chem Toxicol 1998; 36: 347–63. Propylene glycol is rapidly absorbed from the gastrointestinal landin H2 is then converted to the primary prostaglandins pros- 6. Lieberman HD, et al. Allergic contact dermatitis to propolis in a tract. There is evidence of topical absorption when applied to taglandin D2, prostaglandin E2, and prostaglandin F2 , to throm- violin maker. J Am Acad Dermatol 2002; 46 (suppl): S30–S31. damaged skin. boxane A2 (TXA2) via the enzyme thromboxane synthetase, or 7. Giusti F, et al. Sensitization to propolis in 1255 children under- It is extensively metabolised in the liver primarily by oxidation to prostacyclin (PGI2) via the enzyme prostacyclin synthetase. going patch testing. Contact Dermatitis 2004; 51: 255–8. to lactic and pyruvic acid and is also excreted in the urine These products are further metabolised and rapidly inactivated in 8. Walgrave SE, et al. Allergic contact dermatitis from propolis. unchanged. the body. Dermatitis 2005; 16: 209–15. The secondary prostaglandins, prostaglandin A (PGA ), pros- 9. Majiene D, et al. Antifungal and antibacterial activity of propo- ◊ References. 2 2 lis. Curr Nutr Food Sci 2007; 3: 304–8. 1. Yu DK, et al. Pharmacokinetics of propylene glycol in humans taglandin B2 (PGB2), and prostaglandin C2 (PGC2) are derived during multiple dosing regimens. J Pharm Sci 1985; 74: 876–9. from prostaglandin E2, but are formed during extraction and Preparations 2. Speth PAJ, et al. -

Lipocalin-Type Prostaglandin D Synthase Regulates Light-Induced Phase Advance of the Central Circadian Rhythm in Mice
ARTICLE https://doi.org/10.1038/s42003-020-01281-w OPEN Lipocalin-type prostaglandin D synthase regulates light-induced phase advance of the central circadian rhythm in mice Chihiro Kawaguchi et al.# 1234567890():,; We previously showed that mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP) exhibit attenuated light-induced phase shift. To explore the underlying mechan- isms, we performed gene expression analysis of laser capture microdissected suprachias- matic nuclei (SCNs) and found that lipocalin-type prostaglandin (PG) D synthase (L-PGDS) is involved in the impaired response to light stimulation in the late subjective night in PACAP- deficient mice. L-PGDS-deficient mice also showed impaired light-induced phase advance, but normal phase delay and nonvisual light responses. Then, we examined the receptors involved in the response and observed that mice deficient for type 2 PGD2 receptor DP2/ CRTH2 (chemoattractant receptor homologous molecule expressed on Th2 cells) show impaired light-induced phase advance. Concordant results were observed using the selective DP2/CRTH2 antagonist CAY10471. These results indicate that L-PGDS is involved in a mechanism of light-induced phase advance via DP2/CRTH2 signaling. #A list of authors and their affiliations appears at the end of the paper. COMMUNICATIONS BIOLOGY | (2020) 3:557 | https://doi.org/10.1038/s42003-020-01281-w | www.nature.com/commsbio 1 ARTICLE COMMUNICATIONS BIOLOGY | https://doi.org/10.1038/s42003-020-01281-w he mammalian circadian clock system comprises the genes (Fig. 1a). Of these 593 genes, we specifically analyzed genes endogenous master pacemaker located within the supra- that were upregulated (>1.7-fold change) or downregulated (>0.6- T −/− chiasmatic nucleus (SCN) in the hypothalamus and coor- fold change) by light stimulation in PACAP and wild-type mice.