Study of Phosphorus Behaviour in Levitated Silicon-Iron Droplets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
US Schedule for Internet V2
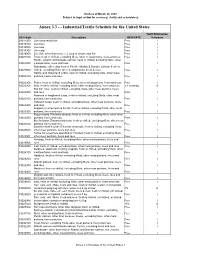
Draft as of March 23, 2007 Subject to legal review for accuracy, clarity and consistency. Annex 3.3 - - Industrial/Textile Schedule for the United States Tariff Elimination US 8 digit Description MFN RATE Schedule 03011000 Live ornamental fish Free I 03019100 Live trout Free I 03019200 Live eels Free I 03019300 Live carp Free I 03019900 Live fish, other than trout, eel, carp or ornamental fish Free I 03021100 Trout, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I Pacific, Atlantic and Danube salmon, fresh or chilled, excluding fillets, other 03021200 meat portions, livers and roes Free I Salmonidae other than trout or Pacific, Atlantic & Danube salmon, fresh or 03021900 chilled, excluding fillets, other meat portions, livers & roes Free I Halibut and Greenland turbot, fresh or chilled, excluding fillets, other meat 03022100 portions, livers and roes Free I 03022200 Plaice, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I 03022300 Sole, fresh or chilled, excluding fillets, other meat portions, livers and roes 1.1 cents/kg A Flat fish, nesi, fresh or chilled, excluding fillets, other meat portions, livers 03022900 and roes Free I Albacore or longfinned tunas, fresh or chilled, excluding fillets, other meat 03023100 portions, livers and roes Free I Yellowfin tunas, fresh or chilled, excluding fillets, other meat portions, livers 03023200 and roes Free I Skipjack or stripe-bellied bonito, fresh or chilled, excluding fillets, other meat 03023300 portions, livers and roes Free -

Binary and Ternary Transition-Metal Phosphides As Hydrodenitrogenation Catalysts
Research Collection Doctoral Thesis Binary and ternary transition-metal phosphides as hydrodenitrogenation catalysts Author(s): Stinner, Christoph Publication Date: 2001 Permanent Link: https://doi.org/10.3929/ethz-a-004378279 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss. ETH No. 14422 Binary and Ternary Transition-Metal Phosphides as Hydrodenitrogenation Catalysts A dissertation submitted to the Swiss Federal Institute of Technology Zurich for the degree of Doctor of Natural Sciences Presented by Christoph Stinner Dipl.-Chem. University of Bonn born February 27, 1969 in Troisdorf (NRW), Germany Accepted on the recommendation of Prof. Dr. Roel Prins, examiner Prof. Dr. Reinhard Nesper, co-examiner Dr. Thomas Weber, co-examiner Zurich 2001 I Contents Zusammenfassung V Abstract IX 1 Introduction 1 1.1 Motivation 1 1.2 Phosphides 4 1.2.1 General 4 1.2.2 Classification 4 1.2.3 Preparation 5 1.2.4 Properties 12 1.2.5 Applications and Uses 13 1.3 Scope of the Thesis 14 1.4 References 16 2 Characterization Methods 1 2.1 FT Raman Spectroscopy 21 2.2 Thermogravimetric Analysis 24 2.3 Temperature-Programmed Reduction 25 2.4 X-Ray Powder Diffractometry 26 2.5 Nitrogen Adsorption 28 2.6 Solid State Nuclear Magnetic Resonance Spectroscopy 28 2.7 Catalytic Test 33 2.8 References 36 3 Formation, Structure, and HDN Activity of Unsupported Molybdenum Phosphide 37 3.1 Introduction -

Download SCOPE Newsletter
With participation of the European Commission: DG GROW and Joint Research Centre Summary of joint European Commission – ESPP webinar th on P4 (phosphorus) Critical Raw Material, 9 July 2020 This ESPP SCOPE Newsletter summarises discussions at the expert webinar jointly organised by ESPP and the European Commission on 9th July, with participation of Contents the leading companies using phosphorus (P4) or its derivatives in Europe, with additional input from the The EU Critical Raw Materials: “Phosphate Rock” and “Phosphorus”........................................................................... 2 independent industry expert Willem Schipper. The content (Fig. i) Overview numbers: from phosphate rock to P4 / P4-derivatives below has been seen by the participants but does not and applications .................................................................................. 3 represent their position, it represents ESPP’s own How is P4 produced? the phosphorus furnace .............. 4 assessment. Photo: P4 furnace ................................................................................ 4 Schematic: P4 manufacture process (simplified) ................................. 4 This webinar aimed to provide expert input to the European Which chemicals critically depend on P4 / P4 derivatives? . 5 Commission’s MSA (Material System Analysis) of the EU dependency on P4 concerns many industry sectors . 5 specific industrial form of “Phosphorus”, P4, as a Critical (Fig. ii) Production via wet-acid or via P4 / P4 derivatives / ‘thermal’ Raw -

Catalog of Standard Reference Materials
NATL INST. OF STAND & TECH Bureau of Standards £-01 Admin. Bldg. AUG 1 8 1970 NBS SPECIAL PUBLICATION 260 NBS JULY 1970 EDITION PUBLICATIONS Catalog of 9TAMDARD "REFERENCE MATERIALS U.S. DEPARTMENT OF COMMERCE National Bureau of Standards - NATIONAL BUREAU OF STANDARDS The National Bureau of Standards 1 was established by an act of Congress March 3, 1901. Today, in addition to serving as the Nation's central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To this end the Bureau conducts research and provides central national services in four broad program areas. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Radiation Research, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and com- merce. The Institute consists of an Office of Measurement Services and the following technical divisions: Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic and Molec- ular Physics—Radio Physics - —Radio Engineering -—Time and Frequency -—Astro- physics - —Cryogenics. -

Other Than Radioactive Ores) Answering to a Description in Heading 2844 Or 2845 Are to Be Classified in Those Headings and in No Other Heading of the Tariff Schedule
)&f1y3X SECTION VI PRODUCTS OF THE CHEMICAL OR ALLIED INDUSTRIES VI-1 Notes 1. (a) Goods (other than radioactive ores) answering to a description in heading 2844 or 2845 are to be classified in those headings and in no other heading of the tariff schedule. (b) Subject to paragraph (a) above, goods answering to a description in heading 2843 or 2846 are to be classified in those headings and in no other heading of this section. 2. Subject to note 1 above, goods classifiable in heading 3004, 3005, 3006, 3212, 3303, 3304, 3305, 3306, 3307, 3506, 3707 or 3808 by reason of being put up in measured doses or for retail sale are to be classified in those headings and in no other heading of the tariff schedule. 3. Goods put up in sets consisting of two or more separate constituents, some or all of which fall in this section and are intended to be mixed together to obtain a product of section VI or VII, are to be classified in the heading appropriate to that product, provided that the constituents are: (a) Having regard to the manner in which they are put up, clearly identifiable as being intended to be used together without first being repacked; (b) Entered together; and (c) Identifiable, whether by their nature or by the relative proportions in which they are present, as being complementary one to another. Additional U.S. Notes 1. In determining the amount of duty applicable to a solution of a single compound in water subject to duty in this section at a specific rate, an allowance in weight or volume, as the case may be, shall be made for the water in excess of any water of crystallization which may be present in the undissolved compound. -

Minerals, Critical Minerals
MINERALS, CRITICAL MINERALS, AND THE U.S. ECONOMY Prepublication Version THIS PREPUBLICATION VERSION OF MINERALS, CRITICAL MINERALS, AND THE U.S. ECONOMY has been provided to the public to facilitate timely access to the committee’s findings. Although the substance of the report is final, editorial changes may be made throughout the text, and citations will be checked prior to publication. The final report will be available through the National Academies Press in the December/January timeframe. MINERALS, CRITICAL MINERALS, AND THE U.S. ECONOMY Committee on Critical Mineral Impacts on the U.S. Economy Committee on Earth Resources Board on Earth Sciences and Resources Division on Earth and Life Studies THE NATIONAL ACADEMIES PRESS Washington, D.C. www.nap.edu Prepublication Version – Subject to Further Editorial Revision THE NATIONAL ACADEMIES PRESS • 500 Fifth Street, N.W. • Washington, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Institute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance. This study was supported by the Department of the Interior / U.S. Geological Survey, under Award No. 06HQGR0204, and by the National Mining Association. The opinions, findings, and conclusions or recommendations contained in this document are those of the authors and do not necessarily reflect the views of the organizations or agencies that provided support for the project. -

(CDR) by CASRN Or Accession Number
List of Chemicals Reported for the 2012 Chemical Data Reporting (CDR) by CASRN or Accession Number For the 2012 CDR, 7,674 unique chemicals were reported by manufacturers (including importers). Chemicals are listed by CAS Registry Number (for non-confidential chemicals) or by TSCA Accession Number (for chemicals listed on the confidential portion of the TSCA Inventory). CASRN or CASRN or ACCESSION ACCESSION NUMBER CA INDEX NAME or GENERIC NAME NUMBER CA INDEX NAME or GENERIC NAME 100016 Benzenamine, 4-nitro- 10042769 Nitric acid, strontium salt (2:1) 10006287 Silicic acid (H2SiO3), potassium salt (1:2) 10043013 Sulfuric acid, aluminum salt (3:2) 1000824 Urea, N-(hydroxymethyl)- 10043115 Boron nitride (BN) 100107 Benzaldehyde, 4-(dimethylamino)- 10043353 Boric acid (H3BO3) 1001354728 4-Octanol, 3-amino- 10043524 Calcium chloride (CaCl2) 100174 Benzene, 1-methoxy-4-nitro- 100436 Pyridine, 4-ethenyl- 10017568 Ethanol, 2,2',2''-nitrilotris-, phosphate (1:?) 10043842 Phosphinic acid, manganese(2+) salt (2:1) 2,7-Anthracenedisulfonic acid, 9,10-dihydro- 100447 Benzene, (chloromethyl)- 10017591 9,10-dioxo-, sodium salt (1:?) 10045951 Nitric acid, neodymium(3+) salt (3:1) 100185 Benzene, 1,4-bis(1-methylethyl)- 100469 Benzenemethanamine 100209 1,4-Benzenedicarbonyl dichloride 100470 Benzonitrile 100210 1,4-Benzenedicarboxylic acid 100481 4-Pyridinecarbonitrile 10022318 Nitric acid, barium salt (2:1) 10048983 Phosphoric acid, barium salt (1:1) 9-Octadecenoic acid (9Z)-, 2-methylpropyl 10049044 Chlorine oxide (ClO2) 10024472 ester Phosphoric acid, -

Theory and Technology of the Phosphorus Extraction from Ferrophosphorus
Modern Applied Science; Vol. 9, No. 6; 2015 ISSN 1913-1844 E-ISSN 1913-1852 Published by Canadian Center of Science and Education Theory and Technology of the Phosphorus Extraction from Ferrophosphorus Serzhanov Galimzhan Mendikarayevich1, Shevko Viktor Mikhaylovich1 &Yeskendirova Marina Mikhaylovna1 1 M. Auezov South Kazakhstan State University, Republic of Kazakhstan Correspondence: Serzhanov Galimzhan Mendikarayevich, M. Auezov South Kazakhstan State University, Republic of Kazakhstan. Received: December 3, 2014 Accepted: December 9, 2014 Online Published: May 30, 2015 doi:10.5539/mas.v9n6p160 URL: http://dx.doi.org/10.5539/mas.v9n6p160 Abstract This article contains the research results of thermodynamic modelling of the chemical interaction of iron phosphides (Fe3P, Fe 2P, FeP and FeP2) with ferrosilicon FeSi2. The results were received by a method of the full thermodynamic analysis using the program HSC-5.1 of the Finnish metallurgical company Outokumpu, which was developed on a principle of the Gibbs energy minimization. The article considers the experimental results concerning to kinetics of the phosphorus extraction from electrothermal ferrophosphorus using ferrosilicon of a grade FS65. In addition, optimum technological parameters of the phosphorus extraction from the industrial ferrophosphorus, containing 26,4 % of phosphorus, and manufacture of a ferroalloy were determined. Keywords: iron phosphides, ferrophosphorus, iron silicides, thermodynamic modelling, kinetics, ferrosilicon, phosphorus 1. Introduction At the electrothermal production of yellow phosphorus from a phosphorite, quartzite and coke mixture, 1 ton of the phosphorus produced gives to 300 kg of a by-product – ferrophosphorus (1). The basic consumer of ferrophosphorus is the ferrous metallurgy, in which ferrophosphorus is used for melting of alloyed phosphorus-containing steels (for example, automatic steels), and iron casting. -

United States Patent (19) 11 Patent Number: 4,794,050 Campbell Et Al
United States Patent (19) 11 Patent Number: 4,794,050 Campbell et al. (45) Date of Patent: Dec. 27, 1988 (54) RESISTANCE WELDING OF GALVANIZED STEEL OTHER PUBLICATIONS 75 Inventors: Donald H. Campbell, Honjo et al., “International Journal of Materials and Niagara-on-the-Lake, Canada; Product Technology" vol. 1 No. 1 pp. 83-114 1986. Gerald L. Evarts, Tonawanda, N.Y. Primary Examiner-John F. Terapane 73) Assignee: Occidental Chemical Corporation, Assistant Examiner-Eric Jorgensen Niagara Falls, N.Y. Attorney, Agent, or Firm-James F. Tao; William G. (21) Appl. No.: 59,298 Gosz 22 Filed: Jun. 8, 1987 57 ABSTRACT A coating for improved resistance welding of galva 51) Int. Cl." ................................................ B22F7/04 nized steel parts or sheets comprises a binder, and a 52 U.S.C. ........................................ as a to a 428/551 metal phosphide pigment, preferably a ferrophosphorus 58 Field of Search ................ 428/551, 555, 556, 621 pigment, having a particle size of from about 0.1 to 56 References Cited about 30 microns. The pigment can also include up to U.S. PATENT DOCUMENTS about 40% by weight of an additional metal such as tin, 4,337,299 6/1982 Van den Bergh .................. 428/551 aluminum or lead, which can be combined with the 4,350,539 9/1982 Torok et al. ........................ 428/555 metal phosphide in pigment form or deposited onto the 4,497,876 2/1985 Kidon .................................. 428/621 surface of the particles. The coating can be applied to 4,524,111 6/1985 Oka et al. ............................ 428/551 either the faying or non-faying surfaces of the galva 4,601,957 7/1986 Fujimoto et al. -

Phosphorus Recovery and Recycling – Closing the Loop
Chem Soc Rev View Article Online TUTORIAL REVIEW View Journal | View Issue Phosphorus recovery and recycling – closing the loop† Cite this: Chem. Soc. Rev.,2021, 50,87 Andrew R. Jupp, ‡*ab Steven Beijer,‡a Ganesha C. Narain,a Willem Schipperc and J. Chris Slootweg *a There is a clear and pressing need to better manage our planet’s resources. Phosphorus is a crucial element for life, but the natural phosphorus cycle has been perturbed to such an extent that humanity faces two dovetailing problems: the dwindling supply of phosphate rock as a resource, and the overabundance of phosphate in water systems leading to eutrophication. This Tutorial Review will explore the current routes to industrial phosphorus compounds, and innovative academic routes towards accessing these same products in a more sustainable manner. It will then describe the many Received 4th September 2020 ways that useful phosphate can be recovered from waste streams, and how it can be recycled and used DOI: 10.1039/d0cs01150a as a resource for new products. Finally, we will briefly discuss the barriers that have thus far prevented Creative Commons Attribution-NonCommercial 3.0 Unported Licence. the widespread adoption of these technologies, and how we can close the loop to establish a modern rsc.li/chem-soc-rev phosphorus cycle. Key learning points (1) Why is phosphorus important and how has the natural phosphorus cycle been disrupted? (2) How are phosphorus-containing compounds currently synthesized? (3) Can these same products be accessed in more environmentally friendly and sustainable ways? (4) How can phosphorus be recovered and recycled? This article is licensed under a (5) What barriers need to be overcome to re-establish the phosphorus cycle? Open Access Article. -

United States Patent (19) 11 Patent Number: 4,906,522 Miller (45) Date of Patent: Mar
United States Patent (19) 11 Patent Number: 4,906,522 Miller (45) Date of Patent: Mar. 6, 1990 (54) COMPOUNDS OF NICKEL, IRON AND 4,690,863 9/1987 Miyoshi et al. ..................... 428/328 PHOSPHORUS 4,728,462 3/1988 Miller et al. ........ ... 252/S19 4,735,864 4/1988 Masumoto et al. ................. 428/606 (75) Inventor: George T. Miller, Lewiston, N.Y. 4,748,080 5/1988 Itozawa ............................... 428/329 73) Assignee: Occidental Chemical Corporation, 4,774,270 9/1988 Sullivan et al. ..................... 252/519 Niagara Falls, N.Y. Primary Examiner-George F. Lesmes Assistant Examiner-Elizabeth M. Cole 21) Appl. No.: 42,195 Attorney, Agent, or Firm-James F. Tao; Richard D. (22 Filed: Apr. 24, 1987 Fuerle 51 Int. Cl." ........................ B32B 27/20; C22B 4/06; (57) ABSTRACT C22C 38/08 52 U.S.C. ................................... 428/323; 7.5/10.63; Compositions of nickel, iron and phosphorus, are pre 75/10.66; 106/456; 106/480; 252/513; 420/94; pared by fusing nickel and ferrophosphorus in an inert 420/119; 428/457; 428/704 atmosphere, with the nickel preferably comprising from 58) Field of Search ................. 420/94, 119, 75/10.63, about 5% to about 70% by weight of the composition. 75/10.66, 113; 252/513, 62.55; 106/456, 480; The fused composition is ground or atomized to form a 428/323 conductive, corrosion resistant pigment, preferably having a particle size of from about 0.1 to about 15 (56) References Cited microns. This pigment can then be incorporated into a U.S. PATENT DOCUMENTS suitable resin binder, optionally with one or more other 2,757,083 7/1956 Story .................................... -
F1y3x SECTION VI PRODUCTS of the CHEMICAL OR
)&f1y3X SECTION VI PRODUCTS OF THE CHEMICAL OR ALLIED INDUSTRIES VI-1 Notes 1. (a) Goods (other than radioactive ores) answering to a description in heading 2844 or 2845 are to be classified in those headings and in no other heading of the tariff schedule. (b) Subject to paragraph (a) above, goods answering to a description in heading 2843 or 2846 are to be classified in those headings and in no other heading of this section. 2. Subject to note 1 above, goods classifiable in heading 3004, 3005, 3006, 3212, 3303, 3304, 3305, 3306, 3307, 3506, 3707 or 3808 by reason of being put up in measured doses or for retail sale are to be classified in those headings and in no other heading of the tariff schedule. 3. Goods put up in sets consisting of two or more separate constituents, some or all of which fall in this section and are intended to be mixed together to obtain a product of section VI or VII, are to be classified in the heading appropriate to that product, provided that the constituents are: (a) Having regard to the manner in which they are put up, clearly identifiable as being intended to be used together without first being repacked; (b) Entered together; and (c) Identifiable, whether by their nature or by the relative proportions in which they are present, as being complementary one to another. Additional U.S. Notes 1. In determining the amount of duty applicable to a solution of a single compound in water subject to duty in this section at a specific rate, an allowance in weight or volume, as the case may be, shall be made for the water in excess of any water of crystallization which may be present in the undissolved compound.