Other Than Radioactive Ores) Answering to a Description in Heading 2844 Or 2845 Are to Be Classified in Those Headings and in No Other Heading of the Tariff Schedule
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

[email protected] +1-703-527-3887 (International) Website
Date of Issue: 17 June 2015 SAFETY DATA SHEET 1. SUBSTANCE AND SOURCE IDENTIFICATION Product Identifier SRM Number: 1877 SRM Name: Beryllium Oxide Powder Other Means of Identification: Not applicable. Recommended Use of This Material and Restrictions of Use This Standard Reference Material (SRM) is intended for use in laboratory analysis and health research for the development and validation of analytical methods and instruments used to determine beryllium, as well as for proficiency testing of laboratories involved in beryllium determinations. A unit of SRM 1877 consists of one bottle containing 20 g of beryllium oxide powder. Company Information National Institute of Standards and Technology Standard Reference Materials Program 100 Bureau Drive, Stop 2300 Gaithersburg, Maryland 20899-2300 Telephone: 301-975-2200 Emergency Telephone ChemTrec: FAX: 301-948-3730 1-800-424-9300 (North America) E-mail: [email protected] +1-703-527-3887 (International) Website: http://www.nist.gov/srm 2. HAZARDS IDENTIFICATION Classification Physical Hazard: Not classified. Health Hazard: Acute Toxicity, Inhalation Category 2 Skin Corrosion/Irritation Category 2 Serious Eye Damage/Irritation Category 2B Skin Sensitization Category 1 Carcinogenicity Category 1A STOT-Repeat Exposure Category 1 Label Elements Symbol Signal Word DANGER Hazard Statement(s) H330 Fatal if inhaled. H315+H320 Causes skin and eye irritation. H317 May cause an allergic skin reaction. H350 May cause lung cancer. H372 Causes damage to lungs through prolonged or repeated inhalation. Precautionary Statement(s) P201 Obtain special instructions before use. P202 Do not handle until all safety precautions have been read and understood. P260 Do not breathe dust. P264 Wash hands thoroughly after handling. -

Exposure Data
BERYLLIUM AND BERYLLIUM eOMPOUNDS Beryllium and beryllium compounds were considered by previous Working Groups, In 1971,1979 and 1987 (lARe, 1972, 1980, 1987a). New data have since become available, and these are included in the present monograph and have been taken into consideration In the evaluation. The agents considered herein Include (a) metallic beryllium, (b) beryllium- aluminium and -copper alloys and (c) some beryllum compounds. 1. Exposure Data 1.1 Chemical and physical data and analysis 1.1.1 Synonyms, trade names and molecular formulae Synonyms, trade names and molecular formulae for beryllium, beryllum-aluminium and -copper alloys and certain beryllium compounds are presented in Thble 1. The list is not exhaustive, nor does it comprise necessarily the most commercially important beryllum- containing substances; rather, it indicates the range of beryllum compounds available. 1. 1.2 Chemical and physical properties of the pure substances Selected chemical and physical properties of beryllium, beryllum-aluminium and -copper alloys and the beryllium compounds covered in this monograph are presented in Thble 2. The French chemist Vauquelin discovered beryllium in 1798 as the oxide, while analysing emerald to prove an analogous composition (Newland, 1984). The metallc element was first isolated in independent experiments by Wöhler (1828) and Bussy (1828), who called it 'glucinium' owing to the sweet taste of its salts; that name is stil used in the French chemical literature. Wöhler's name 'beryllum' was offcially recognized by IUPAe in 1957 (WHO, 1990). The atomic weight and corn mon valence of beryllum were originally the subject of much controversy but were correctly predicted by Mendeleev to be 9 and + 2, respectively (Everest, 1973). -
US Schedule for Internet V2
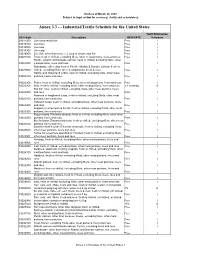
Draft as of March 23, 2007 Subject to legal review for accuracy, clarity and consistency. Annex 3.3 - - Industrial/Textile Schedule for the United States Tariff Elimination US 8 digit Description MFN RATE Schedule 03011000 Live ornamental fish Free I 03019100 Live trout Free I 03019200 Live eels Free I 03019300 Live carp Free I 03019900 Live fish, other than trout, eel, carp or ornamental fish Free I 03021100 Trout, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I Pacific, Atlantic and Danube salmon, fresh or chilled, excluding fillets, other 03021200 meat portions, livers and roes Free I Salmonidae other than trout or Pacific, Atlantic & Danube salmon, fresh or 03021900 chilled, excluding fillets, other meat portions, livers & roes Free I Halibut and Greenland turbot, fresh or chilled, excluding fillets, other meat 03022100 portions, livers and roes Free I 03022200 Plaice, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I 03022300 Sole, fresh or chilled, excluding fillets, other meat portions, livers and roes 1.1 cents/kg A Flat fish, nesi, fresh or chilled, excluding fillets, other meat portions, livers 03022900 and roes Free I Albacore or longfinned tunas, fresh or chilled, excluding fillets, other meat 03023100 portions, livers and roes Free I Yellowfin tunas, fresh or chilled, excluding fillets, other meat portions, livers 03023200 and roes Free I Skipjack or stripe-bellied bonito, fresh or chilled, excluding fillets, other meat 03023300 portions, livers and roes Free -

Federal Law and Vertebrate Pest Control
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Proceedings of the 1st Vertebrate Pest Vertebrate Pest Conference Proceedings Conference (1962) collection February 1962 FEDERAL LAW AND VERTEBRATE PEST CONTROL Justus C. Ward Director, Pesticides Regulation Division, Agricultural Research Service, U.S. Department of Agriculture Follow this and additional works at: https://digitalcommons.unl.edu/vpcone Part of the Environmental Health and Protection Commons Ward, Justus C., "FEDERAL LAW AND VERTEBRATE PEST CONTROL" (1962). Proceedings of the 1st Vertebrate Pest Conference (1962). 25. https://digitalcommons.unl.edu/vpcone/25 This Article is brought to you for free and open access by the Vertebrate Pest Conference Proceedings collection at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Proceedings of the 1st Vertebrate Pest Conference (1962) by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. FEDERAL LAW AND VERTEBRATE PEST CONTROL By: Justus C. Ward, Director, Pesticides Regulation Division, Agricultural Research Service, U.S. Department of Agriculture Presented at the Vertebrate Pest Control Conference, Sacramento, California, February 6 and 7, 1 962 Shortly after the passage of the Federal Insecticide Act of 1910> mammal control specialists in the Bureau of Biological Survey began to consider a similar law to cover the chemicals with which they were concerned. Work on the project went slowly a nd spasmodically, but reached the point of having a Federal Rodenticide Act available for study and possible revision in 1928. At this time, the mammal control chemicals in use were limited to strychnine -- alkaloid and sulphate -arsenic, barium carbonate, th allium sulphate, phosphorus, s odium and calcium cyanide, carbon disulphide, and red squill. -

Toxicological Profile for Beryllium
BERYLLIUM 19 3. HEALTH EFFECTS 3.1 INTRODUCTION The primary purpose of this chapter is to provide public health officials, physicians, toxicologists, and other interested individuals and groups with an overall perspective on the toxicology of beryllium. It contains descriptions and evaluations of toxicological studies and epidemiological investigations and provides conclusions, where possible, on the relevance of toxicity and toxicokinetic data to public health. A glossary and list of acronyms, abbreviations, and symbols can be found at the end of this profile. 3.2 DISCUSSION OF HEALTH EFFECTS BY ROUTE OF EXPOSURE To help public health professionals and others address the needs of persons living or working near hazardous waste sites, the information in this section is organized first by route of exposure (inhalation, oral, and dermal) and then by health effect (death, systemic, immunological, neurological, reproductive, developmental, genotoxic, and carcinogenic effects). These data are discussed in terms of three exposure periods: acute (14 days or less), intermediate (15–364 days), and chronic (365 days or more). Levels of significant exposure for each route and duration are presented in tables and illustrated in figures. The points in the figures showing no-observed-adverse-effect levels (NOAELs) or lowest-observed-adverse-effect levels (LOAELs) reflect the actual doses (levels of exposure) used in the studies. LOAELS have been classified into "less serious" or "serious" effects. "Serious" effects are those that evoke failure in a biological system and can lead to morbidity or mortality (e.g., acute respiratory distress or death). "Less serious" effects are those that are not expected to cause significant dysfunction or death, or those whose significance to the organism is not entirely clear. -

"Beryllium Oxide Digestion Optimization at the Savannah River Site"
"Beryllium Oxide Digestion Optimization at the Savannah River Site" by Davin Jagnandan November, 2002 ==' ¥---- ; A paper submitted to the faculty of the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Master of Public Health in the School of Public Health Abstract Chronic beryllium disease (CBD) is a debilitating lung disorder affecting an estimated 4.6% of past nuclear weapons and beryllium manufacturing workers. The Chronic Beryllium Disease Prevention Program, as set up by the U.S. Department of Energy (DOE), establishes guidelines for maintaining acceptable exposure levels in all beryllium manufacturing facilities. These exposure levels are based on the measuring of total beryllium which includes beryllium metal, beryllium salts, beryllium alloys and beryllium oxide. The most sensitive detection instruments used in industrial hygiene sampling measure beryllium in a solubilized form . Beryllium oxide, in particular, has been shown to resist solubilization unless it is performed in the presence of strong acid(s). This poses a problem for industrial hygienists because beryllium oxide has been shown to have a high level oftoxicity due to its small size and moderate solubility. The current Ip digestion method (EPA 3050B) for all metals at the Savannah River Site 1 involves the use of nitric and hydrochloric acids. It was hypothesized that 1 this method was inadequate for the complete digestion of beryllium oxide. This study examines this issue by comparing method 3050B with two other acid digestion methods. Once the most effective of the three methods was determined, optimization factors such as settling time, heating/reflux time, and hydrogen peroxide addition were examined. -

Study of Phosphorus Behaviour in Levitated Silicon-Iron Droplets
STUDY OF PHOSPHORUS BEHAVIOUR IN LEVITATED SILICON-IRON DROPLETS by Katherine Le A thesis submitted in conformity with the requirements for the degree of Master of Applied Science Department of Materials Science and Engineering University of Toronto © Copyright by Katherine Le 2016 ii Study of Phosphorus Behaviour in Levitated Si-Fe Droplets Katherine Le Master of Applied Science Department of Materials Science and Engineering University of Toronto 2016 Abstract While the treatment of relatively inexpensive ferrosilicon alloys is a potential refining route in order to generate solar grade silicon, phosphorus is one of the more difficult impurities to remove by conventional processing. In this project, electromagnetic levitation was used to investigate the dephosphorization of ferrosilicon alloy droplets exposed to H2-Ar gas mixtures under various experimental conditions including, refining time, temperature (1450°C-1720°C), H2-Ar gas concentrations and flow rate, iron alloying content, and initial phosphorus concentration. Reaction rates increased with higher refining times, temperatures, and H2 gas concentrations. With unknown parameters associated with the kinetics of gas phase reactions, the approach involved comparison of apparent activation energies derived for the chemical reaction and gas diffusion steps of the dephosphorization process. The phosphorus removal rate is thought to be controlled by the interfacial reaction step; further work is required to confirm this conclusion. iii Acknowledgements I would like to express my gratitude and respect to my supervisor, Prof. Alex McLean for the opportunity to work on this research. I am thankful for his guidance, wisdom and encouragement throughout the course of my studies. He is a truly inspiring person, and a great enabler of new learning opportunities. -

Binary and Ternary Transition-Metal Phosphides As Hydrodenitrogenation Catalysts
Research Collection Doctoral Thesis Binary and ternary transition-metal phosphides as hydrodenitrogenation catalysts Author(s): Stinner, Christoph Publication Date: 2001 Permanent Link: https://doi.org/10.3929/ethz-a-004378279 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss. ETH No. 14422 Binary and Ternary Transition-Metal Phosphides as Hydrodenitrogenation Catalysts A dissertation submitted to the Swiss Federal Institute of Technology Zurich for the degree of Doctor of Natural Sciences Presented by Christoph Stinner Dipl.-Chem. University of Bonn born February 27, 1969 in Troisdorf (NRW), Germany Accepted on the recommendation of Prof. Dr. Roel Prins, examiner Prof. Dr. Reinhard Nesper, co-examiner Dr. Thomas Weber, co-examiner Zurich 2001 I Contents Zusammenfassung V Abstract IX 1 Introduction 1 1.1 Motivation 1 1.2 Phosphides 4 1.2.1 General 4 1.2.2 Classification 4 1.2.3 Preparation 5 1.2.4 Properties 12 1.2.5 Applications and Uses 13 1.3 Scope of the Thesis 14 1.4 References 16 2 Characterization Methods 1 2.1 FT Raman Spectroscopy 21 2.2 Thermogravimetric Analysis 24 2.3 Temperature-Programmed Reduction 25 2.4 X-Ray Powder Diffractometry 26 2.5 Nitrogen Adsorption 28 2.6 Solid State Nuclear Magnetic Resonance Spectroscopy 28 2.7 Catalytic Test 33 2.8 References 36 3 Formation, Structure, and HDN Activity of Unsupported Molybdenum Phosphide 37 3.1 Introduction -

Step-By-Step Guide to Better Laboratory Management Practices
Step-by-Step Guide to Better Laboratory Management Practices Prepared by The Washington State Department of Ecology Hazardous Waste and Toxics Reduction Program Publication No. 97- 431 Revised January 2003 Printed on recycled paper For additional copies of this document, contact: Department of Ecology Publications Distribution Center PO Box 47600 Olympia, WA 98504-7600 (360) 407-7472 or 1 (800) 633-7585 or contact your regional office: Department of Ecology’s Regional Offices (425) 649-7000 (509) 575-2490 (509) 329-3400 (360) 407-6300 The Department of Ecology is an equal opportunity agency and does not discriminate on the basis of race, creed, color, disability, age, religion, national origin, sex, marital status, disabled veteran’s status, Vietnam Era veteran’s status or sexual orientation. If you have special accommodation needs, or require this document in an alternate format, contact the Hazardous Waste and Toxics Reduction Program at (360)407-6700 (voice) or 711 or (800) 833-6388 (TTY). Table of Contents Introduction ....................................................................................................................................iii Section 1 Laboratory Hazardous Waste Management ...........................................................1 Designating Dangerous Waste................................................................................................1 Counting Wastes .......................................................................................................................8 Treatment by Generator...........................................................................................................12 -

Toxicological Profile for Barium and Barium Compounds
TOXICOLOGICAL PROFILE FOR BARIUM AND BARIUM COMPOUNDS U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry August 2007 BARIUM AND BARIUM COMPOUNDS ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. BARIUM AND BARIUM COMPOUNDS iii UPDATE STATEMENT A Toxicological Profile for Barium and Barium Compounds, Draft for Public Comment was released in September 2005. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology and Environmental Medicine/Applied Toxicology Branch 1600 Clifton Road NE Mailstop F-32 Atlanta, Georgia 30333 BARIUM AND BARIUM COMPOUNDS iv This page is intentionally blank. v FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for the hazardous substance described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a hazardous substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. -

Chemistry 1000 Lecture 13: the Alkaline Earth Metals
Chemistry 1000 Lecture 13: The alkaline earth metals Marc R. Roussel September 25, 2018 Marc R. Roussel Alkaline earth metals September 25, 2018 1 / 23 Mg{Ra Group 2: The alkaline earth metals Group 2, except maybe Be Soft metals Form M2+ cations Very negative reduction potentials: 2+ − M(aq) + 2e ! M(s) Element Be Mg Ca Sr Ba Ra E◦=V −1:847 −2:356 −2:84 −2:89 −2:92 −2:92 Relatively small 1st and 2nd ionization energies: Element Be Mg Ca Sr Ba Ra −1 I1=kJ mol 899:5 737:7 589:8 549:5 502:9 509:3 −1 I2=kJ mol 1757:1 1450:7 1145:4 1064:2 965:2 979:0 Marc R. Roussel Alkaline earth metals September 25, 2018 2 / 23 Mg{Ra Comparison to alkali metals Physical Properties: Property Na Mg Mohs hardness 0.5 2.5 Density=g cm−3 0.968 1.738 Melting point=◦C 97.72 650 Boiling point=◦C 883 1090 Chemical properties are often similar to those of the alkali metals, but less reactive: Example: Reaction with water: M(s) + 2H2O ! M(OH)2 + H2(g) =) Often has to be done in hot water or with steam Marc R. Roussel Alkaline earth metals September 25, 2018 3 / 23 Mg{Ra Why \alkaline earth" metals? The name \alkaline earth" was originally applied to the oxides of these metals. Earth is a term applied by early chemists to nonmetallic substances which are insoluble in water and remain stable when heated. The alkaline earth metal oxides have these properties. -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved.