Chemical Names and CAS Numbers Final
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Guide to Export Controls
Foreign Affairs, Trade and Affaires étrangères, Commerce et Development Canada Développment Canada A Guide To CANADA’S EXPORT CONTROLS December 2012 Introduction The issuance of export permits is administered by the Export Controls Division (TIE) of Foreign Affairs, Trade and Development Canada (DFATD). TIE provides assistance to exporters in determining if export permits are required. It also publishes brochures and Notices to Exporters that are freely available on request and on our website www.exportcontrols.gc.ca. How to contact us: Export Controls Division (TIE) Foreign Affairs, Trade and Development Canada 111 Sussex Drive Ottawa, Ontario K1A 0G2 Telephone: (613) 996-2387 Facsimile: (613) 996-9933 Email: [email protected] For information on how to apply for an export permit and additional information on export controls please refer to our website. To enquire on the status of an export permit application: Recognized EXCOL users can check the status of an export permit application on-line. Non-recognized users can call (613) 996-2387 or email [email protected] and quote your export permit application identification (ref ID) number. Export Controls Division website: www.exportcontrols.gc.ca This Guide, at time of publication, encompasses the list of items enumerated on the Export Control List (ECL) that are controlled for export in accordance with Canadian foreign policy, including Canada’s participation in multilateral export control regimes and bilateral agreements. Unless otherwise specified, the export controls contained in this Guide apply to all destinations except the United States. Canada’s Export Control List can be found at the Department of Justice website at http://canada.justice.gc.ca/. -

1201: Introduction to Aluminium As an Engineering Material
TALAT Lecture 1201 Introduction to Aluminium as an Engineering Material 23 pages, 26 figures (also available as overheads) Basic Level prepared by M H Jacobs * Interdisciplinary Research Centre in Materials The University of Birmingham, UK Objectives To provide an introduction to metallurgical concepts necessary to understand how structural features of aluminium alloys are influenced by alloy composition, processing and heat treatment, and the basic affects of these parameters on the mechanical properties, and hence engineering applications, of the alloys. It is assumed that the reader has some elementary knowledge of physics, chemistry and mathematics. Date of Issue: 1999 EAA - European Aluminium Association 1201 Introduction to Aluminium as an Engineering Material Contents (26 figures) 1201 Introduction to Aluminium as an Engineering Material _____________________ 2 1201.01. Basic mechanical and physical properties__________________________________ 3 1201.01.01 Background _______________________________________________________________ 3 1201.01.02 Commercially pure aluminium ______________________________________________ 4 1201.02 Crystal structure and defects _____________________________________________ 6 1201.02.01 Crystals and atomic bonding __________________________________________________ 6 1201.02.02 Atomic structure of aluminium ______________________________________________ 8 1201.02.03 Crystal structures _________________________________________________________ 8 1201.02.04 Some comments on crystal structures of materials -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

SAFETY DATA SHEET Revision Date 28.07.2021 According to Regulation (EC) No
Version 7.0 SAFETY DATA SHEET Revision Date 28.07.2021 according to Regulation (EC) No. 1907/2006 Print Date 02.10.2021 GENERIC EU MSDS - NO COUNTRY SPECIFIC DATA - NO OEL DATA SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifiers Product name : Aluminum iodide Product Number : 208493 Brand : Aldrich REACH No. : A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later registration deadline. CAS-No. : 7784-23-8 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses : Laboratory chemicals, Manufacture of substances 1.3 Details of the supplier of the safety data sheet Company : Sigma-Aldrich Inc. 3050 SPRUCE ST ST. LOUIS MO 63103 UNITED STATES Telephone : +1 314 771-5765 Fax : +1 800 325-5052 1.4 Emergency telephone Emergency Phone # : 800-424-9300 CHEMTREC (USA) +1-703- 527-3887 CHEMTREC (International) 24 Hours/day; 7 Days/week SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 Skin corrosion (Sub-category 1B), H314 Serious eye damage (Category 1), H318 For the full text of the H-Statements mentioned in this Section, see Section 16. 2.2 Label elements Labelling according Regulation (EC) No 1272/2008 Aldrich- 208493 Page 1 of 8 The life science business of Merck operates as MilliporeSigma in the US and Canada Pictogram Signal word Danger Hazard statement(s) H314 Causes severe skin burns and eye damage. -

Determination of Aluminium As Oxide
DETERMINATION OF ALUMINIUM AS OXIDE By William Blum CONTENTS Page I. Introduction 515 II. General principles 516 III. Historical 516 IV. Precipitation of aluminium hydroxide. 518 1. Hydrogen electrode studies 518 (a) The method 518 (b) Apparatus and solutions employed 518 (c) Results of hydrogen electrode experiments 519 (d) Conclusions from hydrogen electrode experiments 520 2. Selection of an indicator for denning the conditions of precipita- '. tion . 522 3. Factors affecting the form of the precipitate 524 4. Precipitation in the presence of iron 525 V. Washing the precipitate . 525 VI. Separation from other elements 526 VII. Ignition and weighing of the precipitate 528 1. Hygroscopicity of aluminium oxide 529 2. Temperature and time of ignition 529 3. Effect of ammonium chloride upon the ignition 531 VIII. Procedure recommended 532 IX. Confirmatory experiments 532 X. Conclusions '534 I. INTRODUCTION Although a considerable number of precipitants have been pro- posed for the determination of aluminium, direct precipitation of aluminium hydroxide by means of ammonium hydroxide, fol- lowed by ignition to oxide, is most commonly used, especially if no separation from iron is desired, in which latter case special methods must be employed. While the general principles involved in this determination are extremely simple, it has long been recog- nized that certain precautions in the precipitation, washing, and ignition are necessary if accurate results are to be obtained. While, however, most of these details have been studied and dis- cussed by numerous authors, it is noteworthy that few publica- tions or textbooks have taken account of all the factors. In the 515 ; 516 Bulletin of the Bureau of Standards [Voi.i3 present paper it seems desirable, therefore, to assemble the various recommendations and to consider their basis and their accuracy. -

WO 2015/025175 Al 26 February 2015 (26.02.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/025175 Al 26 February 2015 (26.02.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C09K 5/06 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/GB2014/052580 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 22 August 2014 (22.08.2014) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 13 15098.2 23 August 2013 (23.08.2013) GB (84) Designated States (unless otherwise indicated, for every (71) Applicant: SUNAMP LIMITED [GB/GB]; Unit 1, Satel kind of regional protection available): ARIPO (BW, GH, lite Place, Macmerry, Edinburgh EH33 1RY (GB). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (72) Inventors: BISSELL, Andrew John; C/o SunAmp, Unit TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, 1, Satellite Place, Macmerry, Edinburgh EH33 1RY (GB). -

Physical Properties and Data of Optical Materials
Physical Properties and Data of Optical Materials Moriaki Wakaki Keiei Kudo Takehisa Shibuya Laß) CRC Press ^^ J Taylor &. Francis Group '*-*"' Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business • Table of Contents A AI (Aluminum) 1 AlSb (Aluminium Antimonide) 10 ADP (Ammonium Dihydrogen Phosphate) 16 Sb (Antimony) 21 Arsenic Selenium Glass 26 As (33%) + S (30%) + Br (37%) (Arsenic-Sulfur-Bromine Glass) 28 As2Se3 (Arsenic Tri-Selenide) 30 As2S3 (Arsenic Tri-Sulfide Glass) 33 6 Ba (Barium) 39 BaF2 (Barium Fluoride) 42 BaTi03 (Barium Titanate) 47 Be (Beryllium) 50 BeO (Beryllium Oxide) 54 Bi(Bismuth) 56 B(Boron) 61 C Cd(Cadmium) 65 CdSe (Cadmium Selenide) 70 CdS (Cadmium Sulfide) 75 CdTe (Cadmium Telluride) 82 CaC03 (Calcite) 89 CaF2 (Calcium Fluoride) 96 CaW04 (Calcium Tungstate) 105 CsBr (Cesium Bromide) 108 Csl (Cesium Iodide) 113 Cr(Chromium) 118 Cu(Copper) 122 CuCl (Cuprous Chloride) 128 D Diamond 135 G Ga (Gallium) 139 GaSb (Gallium Antimonide) 142 GaAs (Gallium Arsenide) 149 GaP (Gallium Phosphide) 158 Ge (Germanium) 165 Ge + Se + Te (Germanium-Selenium-Tellurium Glass) 180 Glass 182 Au (Gold) 191 * I In (Indium) 199 InSb (Indium Antimonide) 202 InAs (Indium Arsenide) 212 InP (Indium Phosphide) 218 Ir (Iridium) 224 Fe(Iron) 228 L LaF3 (Lanthanum Fluoride) 233 PbF2 (Lead Fluoride) 236 PbSe (Lead Selenide) 237 PbS (Lead Sulfide) 243 PbTe (Lead Telluride) 251 LiF (Lithium Fluoride) 257 Lucite 266 M Mg (Magnesium) 269 MgF2 (Magnesium Fluoride) 275 Mg2Ge (Magnesium Germanide) 282 MgO (Magnesium -
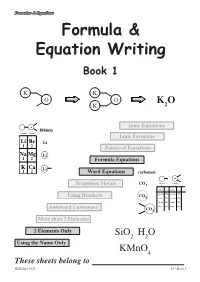
Formula & Equation Writing
Formulae & Equations Formula & Equation Writing Book 1 K K O O K O K 2 Ionic Equations lithium column column 1 2 Ionic Formulae Li Be Li 1 2 Balanced Equations Na Mg Li 1 2 Formula Equations K Ca Li 1 2 Word Equations carbonate valency valency Transition Metals CO3 1 2 name formula name formula ammonium NH4 carbonate CO3 Using Brackets CO 3 cyanide CN chromate CrO4 hydroxide OH sulphate SO4 nitrate NO sulphite SO Awkward Customers 3 3 CO3 More than 2 Elements 2 Elements Only SiO2 H2O Using the Name Only KMnO4 These sheets belong to KHS Sept 2013 page 1 S3 - Book 1 Formulae & Equations What is a Formula ? The formula of a compound tells you two things about the compound :- SiO H O i) which elements are in the compound 2 2 using symbols, and KMnO4 ii) how many atoms of each element are in the compound using numbers. Test Yourself What would be the formula for each of the following? Br H Cl K H C S Al Br O H Cl K H Br Using the Name Only Some compounds have extra information in their carbon monoxide names that allow people to work out and write CO the correct formula. carbon dioxide CO2 The names of the elements appear as usual but dinitrogen tetroxide N O this time the number of each type of atom is 2 4 included using mono- = 1 di- = 12 tri- = 3 tetra- = 4 penta- = 5 hexa- =46 Test Yourself 1 What would be the formula for each of the following? 1. -

United States Patent Office
Patented Feb. 18, 193E _ UNITED STATES PATENT OFFICE PROCESS FOR THE PRODUCTION OF AL ' ' LOYS OF THE ‘EARTH METALS WITH LEAD OR OTHER METALS I ‘Gustaf?ewton Kirsebom, Oslo, Norway, assignor to Calloy limited, London, England, an English . joint-stock company -' . No Drawing. Application December 22, 1932, snug: No. 648,443. \In Great Britain June 11, 9 Claims. (Cl. 75—1) This'invention irelatesto a new or improved pounds of the alkaline earth metals, fox-example method of process for the production of alkaline from the alkaline earth metal silicates. earth metals and alloys thereof with lead or other . In the process as set out above it must be v_ metals such as are hereinafter de?ned. understood that the term.“alkaline earth metals" I ere are certainmetals, especially lead, and includes not only calcium, strontium and barium, ' cadmium which do not readily alloy with alu but also magnesium and beryllium. minium when both are in a molten condition and Where cadmium is employed in place of lead v in the presence of each other, e. g., when molten the procedure is similar and when‘ the alloy of lead is added to a bath of molten aluminium (or cadmium with the alkaline earth metal has been .10 whenlead and aluminium are melted together) formed-on completion of the process-the cad 10 . these two metals will not form an alloy but will mium can be distilled off so that the process af form separate layers which are not substantially fords a ready means of preparing the alkaline ' soluble in each other; and this I utilize in the earth metals in substantially pure condition. -

Alcl3(G)=3Alcl(G) Reaction in the Subhalide Process of Aluminium (Study of Extractive Metallurgy of Aluminium (1))
Equilibrium of the 2Al(l)+AlCl3(g)=3AlCl(g) Reaction in the Subhalide Process of Aluminium (Study of Extractive Metallurgy of Aluminium (1)) By Takeaki Kikuchi*, Toshio Kurosawa* and Testuo Yagihashi* Equilibriumconstants of a fundamental reaction of the aluminium subhalide process, 2Al(l)+AlCl3(g)= 3AlCl(g), were determined by the flow method using argon carrier between 1000℃ and 1250℃. As a result of this experiment, equilibrium constants and standard free energy were obtained by the following equation: The heat of formation and entropy of AlCl(g) obtained from the experimental data and other thermodynamic values were -22,250cal/mot and 48.7cal/mol respectively. By the use of the equilibrium constants, the reaction ratio of aluminium trichloride was calculated at a reduced pressure and in argon carrier, respectively. (Receivedmarch 10, 1964) I. Introduction trichloride supplied to the reaction zone were applied Aluminium is produced by means of the fused salt previously. In this investigation, the latter method electrolysis using alumina obtained mainly from rela- was selected to obtain the equilibrium constants. tively higher grade bauxite. However, another 1. Experimental apparatus extraction method called the Gross or subhalide process has recently been investigated, and some technological The apparatus used in this experiment is shown by or industrialization reports have already been the schematic diagram in Fig. 1. The apparatus con- sists of an argon purifier, aluminium trichloride evapora- published. In this process, crude aluminium alloy is produced tor, reaction tube, and condensing tube of aluminium by reduction of alumina bearing ores with carbonaceous trichloride gas. Argon in a bomb was purified and reducing material such as coke or charcoal in the measured by passing through concentrated sulphuric acid, calcium chloride, soda lime, phosphorus pentoxide first step, and then aluminium trichloride gas is alld magnesium chip hea七ed at 400℃. -

The Formation and Reactivity of I BORON CARBIDE and Related
University of Plymouth PEARL https://pearl.plymouth.ac.uk 04 University of Plymouth Research Theses 01 Research Theses Main Collection 1970 The Formation and Reactivity of BORON CARBIDE and related materials JONES, JAMES ALFRED http://hdl.handle.net/10026.1/1880 University of Plymouth All content in PEARL is protected by copyright law. Author manuscripts are made available in accordance with publisher policies. Please cite only the published version using the details provided on the item record or document. In the absence of an open licence (e.g. Creative Commons), permissions for further reuse of content should be sought from the publisher or author. The Formation and Reactivity of I BORON CARBIDE and related materials A Thesis presented for the Research Degree of DOCTOR OF PHILOSOPHY of the COUNCIL FOR NATIONAL ACADEMIC AWARDS London by JAMES ALFRED JONES Department of Chemistry Plymouth Polytechnic Plymouth, Devon- February^ 1970o F'.V flCCH. i'iC. 1 CLASS, T Shi LJl JoH 13' ABSTRACT 1 The formation of boron carbide, (CBC)*B^^0*^(3^0 is re• viewed with special reference to newer production methods and fabrication techniques. Its crystal structure and the nature of its bonding are discussed in relation to those of other borides and carbides. Information so far available on the sintering of this material is summarised in relation to its reactivity. Sintering into monolithic compoaentBcan only be achieved by hot pressing at pressures between 200 and 300 Kgcm'^ and at temperatures above 2000°C preferably at about 2,300^0 for the most rapid achievement of theoretical density, i.e.