Formula & Equation Writing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SAFETY DATA SHEET Revision Date 28.07.2021 According to Regulation (EC) No
Version 7.0 SAFETY DATA SHEET Revision Date 28.07.2021 according to Regulation (EC) No. 1907/2006 Print Date 02.10.2021 GENERIC EU MSDS - NO COUNTRY SPECIFIC DATA - NO OEL DATA SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifiers Product name : Aluminum iodide Product Number : 208493 Brand : Aldrich REACH No. : A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later registration deadline. CAS-No. : 7784-23-8 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses : Laboratory chemicals, Manufacture of substances 1.3 Details of the supplier of the safety data sheet Company : Sigma-Aldrich Inc. 3050 SPRUCE ST ST. LOUIS MO 63103 UNITED STATES Telephone : +1 314 771-5765 Fax : +1 800 325-5052 1.4 Emergency telephone Emergency Phone # : 800-424-9300 CHEMTREC (USA) +1-703- 527-3887 CHEMTREC (International) 24 Hours/day; 7 Days/week SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 Skin corrosion (Sub-category 1B), H314 Serious eye damage (Category 1), H318 For the full text of the H-Statements mentioned in this Section, see Section 16. 2.2 Label elements Labelling according Regulation (EC) No 1272/2008 Aldrich- 208493 Page 1 of 8 The life science business of Merck operates as MilliporeSigma in the US and Canada Pictogram Signal word Danger Hazard statement(s) H314 Causes severe skin burns and eye damage. -

Nitric Acid Plants (PDF)
Office of Air and Radiation December 2010 AVAILABLE AND EMERGING TECHNOLOGIES FOR REDUCING GREENHOUSE GAS EMISSIONS FROM THE NITRIC ACID PRODUCTION INDUSTRY Available and Emerging Technologies for Reducing Greenhouse Gas Emissions from the Nitric Acid Production Industry Prepared by the Sector Policies and Programs Division Office of Air Quality Planning and Standards U.S. Environmental Protection Agency Research Triangle Park, North Carolina 27711 December 2010 Table of Contents Abbreviations and Acronyms p.1 I Introduction /Purpose of this Document p.2 II. Description of the Nitric Acid Production Process p.3 A. Weak Nitric Acid Production p.3 B. High-Strength Nitric Acid Production p.6 III. N2O Emissions and Nitric Acid Production Process p. 7 IV. Summary of Control Measures p.9 A. Primary Controls p.10 B. Secondary Control p.11 C. Tertiary Controls p.13 D. Selective Catalytic Reduction p.16 V. Other Greenhouse Gas Emissions p.16 VI. Energy Efficiency Improvements p.17 VII. EPA Contacts p.19 VIII. References p.20 Appendix A – Southeast Idaho Energy p.23 Appendix B - US Nitric Acid Plants p.24 Appendix C – CDM Monitoring Reports p.25 Abbreviations and Acronyms atm Pressure in atmospheres BACT Best Available Control Technologies Btu British Thermal Unit Btu/lb Btu per lb of 100% nitric acid CAR Climate Action Reserve CDM Clean Development Mechanism CHP Combined Heat and Power CO2 Carbon dioxide CO2e Carbon dioxide equivalent EU European Union GHG Greenhouse Gases H2 Hydrogen IPCC Intergovernmental Panel on Climate Change IPPC Industrial Pollution Prevention and Control JI Joint Implementation kg N2O/tonne kilograms of N2O per tonne of 100% nitric acid kg CO2e/tonne kilograms of CO2e per tonne of 100% nitric acid lb N2O/ton pounds of N2O per ton of 100% nitric acid N2 Nitrogen NH3 Ammonia NO Nitric oxide NO2 Nitrogen dioxide NOx Nitrogen oxides NSCR Nonselective Catalytic Reduction N2 O4 Nitrogen tetraoxide N2O Nitrous oxide O2 Oxygen PSD Prevention of Significant Deterioration SCR Selective Catalytic Reduction TPD Tons per day 1 I. -

Journal-2019-06
INTELLECTUAL PROPERTY REGISTRY OF SAMOA (IPROS) JOURNAL 2019/06 Publication Date – 4 December 2019 GENERAL INFORMATION The Public is hereby notified that the following persons have applied for the Registration of the Trade Marks as set out in Section 50 of the Intellectual Property Act 2011. Any person who wishes to oppose the registration of any of the under-mentioned Trade Marks, must give notice to the Registrar of Trade Marks, c/- Ministry of Commerce, Industry and Labour, Apia, Samoa within three (3) months from the date of this publication. The notice must be in writing and must include a statement of the grounds of opposition. Each notice of opposition must be accompanied by a filing fee of WST$300. Any person who has any doubts as to whether he/she may be affected by the registration of the Trade Mark in Samoa should consult his/her Solicitor immediately. For any enquiries regarding Trade Marks, please contact the Registry of Intellectual Property at the following telephone number (+685) 20441 or email [email protected] Faafetai, Registrar of Intellectual Property Accepted Trade Marks – Direct Filing (NEWSPAPER VERSION) Application No. : WS/T/7350 Representation of Mark Filing Date : 12 September, 2018 Priority Date : N/A Proprietor/Address : NEST CONSTRUCTION COMPANY LTD Vaitele Uta, Samoa Class(es) 37 Application No. : WS/T/7361 Representation of Mark Filing Date : 24 September, 2018 Priority Date : N/A Proprietor/Address : NIKE INNOVATE C.V. One Bowerman Drive, Beaverton, Oregon 97005--6453, United States of America Class(es) 18 and 25 Application No. : WS/T/7464 Representation of Mark Filing Date : 29 July, 2019 Priority Date : 04/03/2019 Proprietor/Address : REIGN BEVERAGE COMPANY LLC 1547 N. -
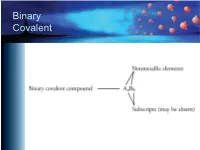
Binary Covalent Common Names
Binary Covalent Common Names – H2O, water – NH3, ammonia – CH4, methane – C2H6, ethane – C3H8, propane – C4H10, butane – C5H12, pentane – C6H14, hexane Naming Binary Covalent CompounDs • If the subscript for the first element is greater than one, inDicate the subscript with a prefix. – We Do not write mono- on the first name. – Leave the "a" off the enD of the prefixes that enD in "a" anD the “o” off of mono- if they are placeD in front of an element that begins with a vowel (oxygen or ioDine). Prefixes mon(o) hex(a) di hept(a) tri oct(a) tetr(a) non(a) pent(a) dec(a) Nitrogen OxiDe Names • N2O3 – name starts with di • N2O5 – name starts with di • NO2 – no initial prefix • NO – no initial prefix Naming Binary Covalent CompounDs • Follow the prefix with the name of the first element in the formula. – N2O3 – dinitrogen – N2O3 – dinitrogen – NO2 – nitrogen – NO – nitrogen Naming Binary Covalent CompounDs • Write a prefix to inDicate the subscript for the seconD element. (Remember to leave the “o” off of mono- anD the “a” off of the prefixes that enD in “a” when they are placeD in front of a name that begins with a vowel.) – N2O3 – dinitrogen tri – N2O5 – dinitrogen pent – NO2 – nitrogen di – NO – nitrogen mon Naming Binary Covalent CompounDs • Write the root of the name of the seconD symbol in the formula. (See the next sliDe.) – N2O3 – dinitrogen triox – N2O5 – dinitrogen pentox – NO2 – nitrogen diox – NO – nitrogen monox Roots of Nonmetals H hydr- F fluor- C carb- Cl chlor- N nitr- Br brom- P phosph- I iod- O ox- S sulf- Se selen- Naming Binary Covalent CompounDs • AdD -ide to the enD of the name. -

(12) Patent Application Publication (10) Pub. No.: US 2015/0175532 A1 Mudduluru Et Al
US 2015O175532A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0175532 A1 Mudduluru et al. (43) Pub. Date: Jun. 25, 2015 (54) PROCESS FOR PREPARATION OF (30) Foreign Application Priority Data 2,3-DIHYDROXY BENZONITRILE Jul. 19, 2012 (IN) ........................... 2926/CHFA2012 (71) Applicant: Laurus Labs Private Limited, Hyderabad (IN) Publication Classification (72) Inventors: Hari Krishna Mudduluru, Hyderabad (51) Int. Cl (IN); Shankar Madhavaram, we Hyderabad (IN) C07C 253/30 (2006.01) (52) U.S. Cl. (73) Assignee: Laurus Labs Private Limited, CPC .................................... C07C 253/30 (2013.01) Hyderabad (IN) (21) Appl. No.: 14/415,527 (57)57 ABSTRACT 1-1. The present invention relates to one pot process for the prepa (22) PCT Filed: Jul.18, 2013 ration of 2,3-dihydroxybenzonitrile from 2,3-dialkoxyben (86). PCT No.: PCT/N2O13AOOO447 Zoic acid without prior isolation of the intermediates. Further the invention relates to the preparation of 2,3-dihydroxyben S371 (c)(1), Zonitrile by dealkylation of 2,3-dialkoxybenzonitrile using (2) Date: Jan. 16, 2015 Suitable aluminum salt-amine complex. US 2015/0175532 A1 Jun. 25, 2015 PROCESS FOR PREPARATION OF 0006. Several reviews have been described for deprotec 2,3-DIHYDROXY BENZONITRILE tion of phenolic ethers. For example, phenolic methyl ethers have deprotected to remove the methyl moiety using hydro PRIORITY gen halide Such as hydrogen chloride or hydrogen bromide under highly acidic conditions; highly dark colored products 0001. This application claims the benefit under Indian Provisional Application No. 2926/CHF/2012 filed 19 Jul. formed in these reactions. In addition the phenolic com 2012 and entitled AN IMPROVED PROCESS FOR THE pounds react further with the halogen compounds used thus PREPARATION OF 2,3-DIHYDROXY BENZONITRILE”, setting a major drawback on this route. -

Chemical Formula of Binary Ionic Compounds – Sheet 1 the Combining Power Or Valency of Silver Is Always 1
Chemical Formula of Binary Ionic Compounds – Sheet 1 The combining power or valency of silver is always 1. All other transition metals are 2 unless otherwise indicated. No. Binary compound Formula No. Binary compound Formula 1 potassium fluoride 26 calcium sulfide 2 calcium chloride 27 lithium bromide 3 barium bromide 28 nickel sulfide 4 silver sulfide 29 zinc phosphide 5 aluminium iodide 30 barium iodide 6 potassium iodide 31 caesium chloride 7 lead(IV) oxide 32 copper bromide 8 zinc nitride 33 sodium nitride 9 silver iodide 34 silver chloride 10 barium fluoride 35 sodium hydride 11 lead(II) iodide 36 potassium nitride 12 silver fluoride 37 cobalt chloride 13 sodium sulfide 38 magnesium sulfide 14 sodium bromide 39 potassium chloride 15 calcium oxide 40 calcium bromide 16 zinc fluoride 41 iron(III) oxide 17 strontium phosphide 42 aluminium fluoride 18 barium sulfide 43 magnesium bromide 19 aluminium oxide 44 iron(III) chloride 20 aluminium chloride 45 barium nitride 21 aluminium sulfide 46 sodium fluoride 22 lead(II) oxide 47 lithium fluoride 23 barium chloride 48 lithium iodide 24 copper chloride 49 lithium hydride 25 barium phosphide 50 potassium oxide “Aluminum” and “cesium” are commonly used alternative spellings for "aluminium" and "caesium that are used in the US. May be freely copied for educational use. ©www.chemicalformula.org Chemical Formula of Binary Ionic Compounds – Sheet 2 The combining power or valency of silver is always 1. All other transition metals are 2 unless otherwise indicated. No. Binary compound Formula No. -

Ep 1831144 B1
(19) & (11) EP 1 831 144 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07C 37/50 (2006.01) C07C 39/17 (2006.01) 17.08.2011 Bulletin 2011/33 C07C 39/26 (2006.01) (21) Application number: 05816593.7 (86) International application number: PCT/HU2005/000128 (22) Date of filing: 07.12.2005 (87) International publication number: WO 2006/061666 (15.06.2006 Gazette 2006/24) (54) A NEW PROCESS FOR THE PREPARATION OF PHENOLIC HYDROXY-SUBSTITUTED COMPOUNDS NEUES VERFAHREN ZUR HERSTELLUNG PHENOLISCHER HYDROXY SUBSTITUIERTER VERBINDUNGEN PROCEDE D’ELABORATION DE COMPOSES PHENOLIQUES A SUBSTITUTION HYDROXY (84) Designated Contracting States: • BORBÉLY, László AT BE BG CH CY CZ DE DK EE ES FI FR GB GR H-2510 Dorog (HU) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • HAÁSZ, Ferenc SK TR H-2500 Esztergom (HU) Designated Extension States: • JEKKEL, Péter AL BA HR MK YU H-2509 Esztergom-Kertváros (HU) (30) Priority: 08.12.2004 HU 0402530 (74) Representative: HOFFMANN EITLE 11.11.2005 HU 0501044 Patent- und Rechtsanwälte Arabellastraße 4 (43) Date of publication of application: 81925 München (DE) 12.09.2007 Bulletin 2007/37 (56) References cited: (73) Proprietor: Richter Gedeon Nyrt. US-A- 4 339 614 1103 Budapest (HU) • MANABU NODE ET.AL.: "Hard acid and soft (72) Inventors: nucleophile system. 2. Demethylation of methyl • VASS, András ethers of alcohol and phenol with an aluminium H-8200 Veszprém (HU) halide-thiolsystem" J. ORG. CHEM., vol. 45, 1980, • DUDÁS, József pages 4275-4277, XP002379759 cited in the H-8200 Veszprém (HU) application Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Chemical Resistance Table
CHEMICAL RESISTANCE TABLE Things to consider when choosing a hose • This chemical resistance table indicates if the inner tube of the hose is resistant to specific materials/chemicals throughout different temperatures. • Some materials/chemicals can change color in contact with the hose. If it´s important to have the color unaffected, we recommend you to contact Hydroscand. • For food products the table only indicates whether the inner tube is resistant to the product. This doesn’t automatically significate that the inner tube is approved for food products. • Abrasion, friction and mechanical influence can increase the chemicals aggression and therefore decrease the durability of the hose. • All values in the table are exclusively for the transport of media. • NB! Materials can change color in contact with other kind of materials. • Outer stress is always an important factor to the durability of the hose. NB! This resistance table should be considered as an indication and not a guarantee. International material codes Rubber Plasic NR Natural rubber P.T.F.E. Polytetraflouro ethylene (Teflon®) SBR Styrene butadiene rubber PP Polypropylene NBR Nitrile rubber UPE Ultra high molekular weight EPDM Ethylene-propylene rubber polyethylene IIR Butyl rubber XLPE Cross linked polyethylene (PEX) CR Chloroprene rubber (Neopren) PU Polyurethane CSM Chlorosulfonated polyethylene PE Polyester platsic (Elastomer) rubber (Hypalon) PA Polyamide (Nylon) PVC Polyvinylchloride How to read the table Fitness grade A Good to excelent B Acceptable for limited use -

Modeling the Vapor – Liquid Equilibrium and Association
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Open Archive Toulouse Archive Ouverte MODELING THE VAPOR – LIQUID EQUILIBRIUM AND ASSOCIATION OF NITROGEN DIOXIDE / DINITROGEN TETROXIDE AND ITS MIXTURES WITH CARBON DIOXIDE A. Belkadi 1,2 , F. Llovell 3, V. Gerbaud 1*, L. F. Vega 2,3*,+ 1Université de Toulouse, LGC (Laboratoire de Génie Chimique), CNRS, INP, UPS, 5 rue Paulin Talabot, F-31106 Toulouse Cedex 01 – France 2MATGAS Research Center, Campus UAB. 08193 Bellaterra, Barcelona Spain 3Institut de Ciència de Materials de Barcelona, (ICMAB-CSIC), Consejo Superior de Investigaciones Científicas, Campus de la UAB, 08193 Bellaterra, Barcelona, Spain * Corresponding authors: [email protected], [email protected]. +Present address: Carburos Metálicos-Grup Air Products. C/ Aragón 300. 08009 Barcelona. Spain. 1 Abstract We have used in this work the crossover soft-SAFT equation of state to model nitrogen dioxide/dinitrogen tetraoxide (NO 2/N 2O4), carbon dioxide (CO 2) and their mixtures. The prediction of the vapor – liquid equilibrium of this mixture is of utmost importance to correctly assess the NO 2 monomer amount that is the oxidizing agent of vegetal macromolecules in the CO 2 + NO 2 / N 2O4 reacting medium under supercritical conditions. The quadrupolar effect was explicitly considered when modeling carbon dioxide, enabling to obtain an excellent description of the vapor-liquid equilibria diagrams. NO 2 was modeled as a self associating molecule with a single association site to account for the strong associating character of the NO 2 molecule. Again, the vapor- liquid equilibrium of NO 2 was correctly modeled. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Nitrogen Oxides (Nox), Why and How They Are Controlled
United States Office of Air Quality EPA 456/F-99-006R Environmental Protection Planning and Standards November 1999 Agency Research Triangle Park, NC 27711 Air EPA-456/F-99-006R November 1999 Nitrogen Oxides (NOx), Why and How They Are Controlled Prepared by Clean Air Technology Center (MD-12) Information Transfer and Program Integration Division Office of Air Quality Planning and Standards U.S. Environmental Protection Agency Research Triangle Park, North Carolina 27711 DISCLAIMER This report has been reviewed by the Information Transfer and Program Integration Division of the Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents of this report reflect the views and policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products is not intended to constitute endorsement or recommendation for use. Copies of this report are available form the National Technical Information Service, U.S. Department of Commerce, 5285 Port Royal Road, Springfield, Virginia 22161, telephone number (800) 553-6847. CORRECTION NOTICE This document, EPA-456/F-99-006a, corrects errors found in the original document, EPA-456/F-99-006. These corrections are: Page 8, fourth paragraph: “Destruction or Recovery Efficiency” has been changed to “Destruction or Removal Efficiency;” Page 10, Method 2. Reducing Residence Time: This section has been rewritten to correct for an ambiguity in the original text. Page 20, Table 4. Added Selective Non-Catalytic Reduction (SNCR) to the table and added acronyms for other technologies. Page 29, last paragraph: This paragraph has been rewritten to correct an error in stating the configuration of a typical cogeneration facility. -

The Chemistry of Solvated Nitric Oxide
The Chemistry of Solvated Nitric Oxide: As the Free Radical and as Super-saturated Dinitrogen Trioxide Solutions By Kristopher A. Rosadiuk March, 2015 A thesis submitted to McGill University in partial fulfillment of the requirements in the degree of: DOCTORATE OF PHILOSOPHY Department of Chemistry, Faculty of Science McGill University Montreal, Quebec, Canada © Kristopher Rosadiuk, 2015. Abstract The unusual behaviour of the mid-oxidation state nitrogen oxides, nitric oxide (NO) and dinitrogen trioxide (N2O3), are explored in solution. Nitric oxide is shown to catalyze the cis-trans isomerizations of diazo species in aqueous and organic solutions, and a model is presented by which this proceeds by spin catalysis, making use of the unpaired electron of NO to permit access to triplet patways. Five diazo compounds are tested and compared to stilbene, which is not found to isomerize under these conditions. Dinitrogen trioxide is found to form easily in organic solvents, which stabilize the molecule even above room temperature. Solutions can be formed at chemically useful concentrations and levels of purity, and this result is compared with the sparse literature concerning this phenomenon. The chemistry of these solutions at 0˚C is surveyed extensively, with 23 distinct organic reactions and 15 inorganic reactions being described. The first reported room temperature adduct of dinitrogen trioxide is presented, as well as novel syntheses for nitrosyl chloride and nitrosylsulfuric acid. X-ray structures are also given for a previously reported benzoquinone-phenol adduct, as well as a new mixed valent-mercury nitride salt of the form Hg4N4O9. Résumé Le comportement inhabituel des oxydes d'azote aux états d’oxydations moyens, comme l’oxyde nitrique (NO) et le trioxyde dinitrique (N2O3), est exploré en solution.