Chemical Resistance Table
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SAFETY DATA SHEET Revision Date 28.07.2021 According to Regulation (EC) No
Version 7.0 SAFETY DATA SHEET Revision Date 28.07.2021 according to Regulation (EC) No. 1907/2006 Print Date 02.10.2021 GENERIC EU MSDS - NO COUNTRY SPECIFIC DATA - NO OEL DATA SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifiers Product name : Aluminum iodide Product Number : 208493 Brand : Aldrich REACH No. : A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later registration deadline. CAS-No. : 7784-23-8 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses : Laboratory chemicals, Manufacture of substances 1.3 Details of the supplier of the safety data sheet Company : Sigma-Aldrich Inc. 3050 SPRUCE ST ST. LOUIS MO 63103 UNITED STATES Telephone : +1 314 771-5765 Fax : +1 800 325-5052 1.4 Emergency telephone Emergency Phone # : 800-424-9300 CHEMTREC (USA) +1-703- 527-3887 CHEMTREC (International) 24 Hours/day; 7 Days/week SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 Skin corrosion (Sub-category 1B), H314 Serious eye damage (Category 1), H318 For the full text of the H-Statements mentioned in this Section, see Section 16. 2.2 Label elements Labelling according Regulation (EC) No 1272/2008 Aldrich- 208493 Page 1 of 8 The life science business of Merck operates as MilliporeSigma in the US and Canada Pictogram Signal word Danger Hazard statement(s) H314 Causes severe skin burns and eye damage. -
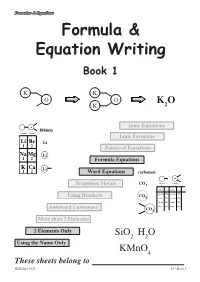
Formula & Equation Writing
Formulae & Equations Formula & Equation Writing Book 1 K K O O K O K 2 Ionic Equations lithium column column 1 2 Ionic Formulae Li Be Li 1 2 Balanced Equations Na Mg Li 1 2 Formula Equations K Ca Li 1 2 Word Equations carbonate valency valency Transition Metals CO3 1 2 name formula name formula ammonium NH4 carbonate CO3 Using Brackets CO 3 cyanide CN chromate CrO4 hydroxide OH sulphate SO4 nitrate NO sulphite SO Awkward Customers 3 3 CO3 More than 2 Elements 2 Elements Only SiO2 H2O Using the Name Only KMnO4 These sheets belong to KHS Sept 2013 page 1 S3 - Book 1 Formulae & Equations What is a Formula ? The formula of a compound tells you two things about the compound :- SiO H O i) which elements are in the compound 2 2 using symbols, and KMnO4 ii) how many atoms of each element are in the compound using numbers. Test Yourself What would be the formula for each of the following? Br H Cl K H C S Al Br O H Cl K H Br Using the Name Only Some compounds have extra information in their carbon monoxide names that allow people to work out and write CO the correct formula. carbon dioxide CO2 The names of the elements appear as usual but dinitrogen tetroxide N O this time the number of each type of atom is 2 4 included using mono- = 1 di- = 12 tri- = 3 tetra- = 4 penta- = 5 hexa- =46 Test Yourself 1 What would be the formula for each of the following? 1. -

Chlorine Dioxide Gas Treatment of Cantaloupe and Residue Analysis Simran Kaur Purdue University
Purdue University Purdue e-Pubs Open Access Theses Theses and Dissertations 2013 Chlorine Dioxide Gas Treatment of Cantaloupe and Residue Analysis Simran Kaur Purdue University Follow this and additional works at: https://docs.lib.purdue.edu/open_access_theses Part of the Food Science Commons Recommended Citation Kaur, Simran, "Chlorine Dioxide Gas Treatment of Cantaloupe and Residue Analysis" (2013). Open Access Theses. 47. https://docs.lib.purdue.edu/open_access_theses/47 This document has been made available through Purdue e-Pubs, a service of the Purdue University Libraries. Please contact [email protected] for additional information. CHLORINE DIOXIDE GAS TREATMENT OF CANTALOUPE AND RESIDUE ANALYSIS A Thesis Submitted to the Faculty of Purdue University by Simran Kaur In Partial Fulfillment of the Requirements for the Degree of Master of Science December 2013 Purdue University West Lafayette, Indiana ii Two cantaloupes were in love. One said to the other, “Let’s run away together!” The other one said, “No. We cantelope.” iii ACKNOWLEDGEMENTS I would like to thank Dr. Mark Morgan for giving me the opportunity to work on this project. I truly appreciate his support, encouragement and guidance and have thoroughly enjoyed every moment. I would also like to thank my committee members, Dr. Mario Ferruzzi and Dr. Peter Hirst for their suggestions, feedback and comments. Thank you to Ben Paxson for his help with equipment and also to Dr. Applegate for the lab space to do my project. I am also thankful for the help and support from all my friends, old and new, close and far, for their support and companionship throughout my graduate studies, especially my dear friend Nadra Guizani. -

Journal-2019-06
INTELLECTUAL PROPERTY REGISTRY OF SAMOA (IPROS) JOURNAL 2019/06 Publication Date – 4 December 2019 GENERAL INFORMATION The Public is hereby notified that the following persons have applied for the Registration of the Trade Marks as set out in Section 50 of the Intellectual Property Act 2011. Any person who wishes to oppose the registration of any of the under-mentioned Trade Marks, must give notice to the Registrar of Trade Marks, c/- Ministry of Commerce, Industry and Labour, Apia, Samoa within three (3) months from the date of this publication. The notice must be in writing and must include a statement of the grounds of opposition. Each notice of opposition must be accompanied by a filing fee of WST$300. Any person who has any doubts as to whether he/she may be affected by the registration of the Trade Mark in Samoa should consult his/her Solicitor immediately. For any enquiries regarding Trade Marks, please contact the Registry of Intellectual Property at the following telephone number (+685) 20441 or email [email protected] Faafetai, Registrar of Intellectual Property Accepted Trade Marks – Direct Filing (NEWSPAPER VERSION) Application No. : WS/T/7350 Representation of Mark Filing Date : 12 September, 2018 Priority Date : N/A Proprietor/Address : NEST CONSTRUCTION COMPANY LTD Vaitele Uta, Samoa Class(es) 37 Application No. : WS/T/7361 Representation of Mark Filing Date : 24 September, 2018 Priority Date : N/A Proprietor/Address : NIKE INNOVATE C.V. One Bowerman Drive, Beaverton, Oregon 97005--6453, United States of America Class(es) 18 and 25 Application No. : WS/T/7464 Representation of Mark Filing Date : 29 July, 2019 Priority Date : 04/03/2019 Proprietor/Address : REIGN BEVERAGE COMPANY LLC 1547 N. -

(12) Patent Application Publication (10) Pub. No.: US 2015/0175532 A1 Mudduluru Et Al
US 2015O175532A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0175532 A1 Mudduluru et al. (43) Pub. Date: Jun. 25, 2015 (54) PROCESS FOR PREPARATION OF (30) Foreign Application Priority Data 2,3-DIHYDROXY BENZONITRILE Jul. 19, 2012 (IN) ........................... 2926/CHFA2012 (71) Applicant: Laurus Labs Private Limited, Hyderabad (IN) Publication Classification (72) Inventors: Hari Krishna Mudduluru, Hyderabad (51) Int. Cl (IN); Shankar Madhavaram, we Hyderabad (IN) C07C 253/30 (2006.01) (52) U.S. Cl. (73) Assignee: Laurus Labs Private Limited, CPC .................................... C07C 253/30 (2013.01) Hyderabad (IN) (21) Appl. No.: 14/415,527 (57)57 ABSTRACT 1-1. The present invention relates to one pot process for the prepa (22) PCT Filed: Jul.18, 2013 ration of 2,3-dihydroxybenzonitrile from 2,3-dialkoxyben (86). PCT No.: PCT/N2O13AOOO447 Zoic acid without prior isolation of the intermediates. Further the invention relates to the preparation of 2,3-dihydroxyben S371 (c)(1), Zonitrile by dealkylation of 2,3-dialkoxybenzonitrile using (2) Date: Jan. 16, 2015 Suitable aluminum salt-amine complex. US 2015/0175532 A1 Jun. 25, 2015 PROCESS FOR PREPARATION OF 0006. Several reviews have been described for deprotec 2,3-DIHYDROXY BENZONITRILE tion of phenolic ethers. For example, phenolic methyl ethers have deprotected to remove the methyl moiety using hydro PRIORITY gen halide Such as hydrogen chloride or hydrogen bromide under highly acidic conditions; highly dark colored products 0001. This application claims the benefit under Indian Provisional Application No. 2926/CHF/2012 filed 19 Jul. formed in these reactions. In addition the phenolic com 2012 and entitled AN IMPROVED PROCESS FOR THE pounds react further with the halogen compounds used thus PREPARATION OF 2,3-DIHYDROXY BENZONITRILE”, setting a major drawback on this route. -

Chemical Formula of Binary Ionic Compounds – Sheet 1 the Combining Power Or Valency of Silver Is Always 1
Chemical Formula of Binary Ionic Compounds – Sheet 1 The combining power or valency of silver is always 1. All other transition metals are 2 unless otherwise indicated. No. Binary compound Formula No. Binary compound Formula 1 potassium fluoride 26 calcium sulfide 2 calcium chloride 27 lithium bromide 3 barium bromide 28 nickel sulfide 4 silver sulfide 29 zinc phosphide 5 aluminium iodide 30 barium iodide 6 potassium iodide 31 caesium chloride 7 lead(IV) oxide 32 copper bromide 8 zinc nitride 33 sodium nitride 9 silver iodide 34 silver chloride 10 barium fluoride 35 sodium hydride 11 lead(II) iodide 36 potassium nitride 12 silver fluoride 37 cobalt chloride 13 sodium sulfide 38 magnesium sulfide 14 sodium bromide 39 potassium chloride 15 calcium oxide 40 calcium bromide 16 zinc fluoride 41 iron(III) oxide 17 strontium phosphide 42 aluminium fluoride 18 barium sulfide 43 magnesium bromide 19 aluminium oxide 44 iron(III) chloride 20 aluminium chloride 45 barium nitride 21 aluminium sulfide 46 sodium fluoride 22 lead(II) oxide 47 lithium fluoride 23 barium chloride 48 lithium iodide 24 copper chloride 49 lithium hydride 25 barium phosphide 50 potassium oxide “Aluminum” and “cesium” are commonly used alternative spellings for "aluminium" and "caesium that are used in the US. May be freely copied for educational use. ©www.chemicalformula.org Chemical Formula of Binary Ionic Compounds – Sheet 2 The combining power or valency of silver is always 1. All other transition metals are 2 unless otherwise indicated. No. Binary compound Formula No. -

Ep 1831144 B1
(19) & (11) EP 1 831 144 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07C 37/50 (2006.01) C07C 39/17 (2006.01) 17.08.2011 Bulletin 2011/33 C07C 39/26 (2006.01) (21) Application number: 05816593.7 (86) International application number: PCT/HU2005/000128 (22) Date of filing: 07.12.2005 (87) International publication number: WO 2006/061666 (15.06.2006 Gazette 2006/24) (54) A NEW PROCESS FOR THE PREPARATION OF PHENOLIC HYDROXY-SUBSTITUTED COMPOUNDS NEUES VERFAHREN ZUR HERSTELLUNG PHENOLISCHER HYDROXY SUBSTITUIERTER VERBINDUNGEN PROCEDE D’ELABORATION DE COMPOSES PHENOLIQUES A SUBSTITUTION HYDROXY (84) Designated Contracting States: • BORBÉLY, László AT BE BG CH CY CZ DE DK EE ES FI FR GB GR H-2510 Dorog (HU) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • HAÁSZ, Ferenc SK TR H-2500 Esztergom (HU) Designated Extension States: • JEKKEL, Péter AL BA HR MK YU H-2509 Esztergom-Kertváros (HU) (30) Priority: 08.12.2004 HU 0402530 (74) Representative: HOFFMANN EITLE 11.11.2005 HU 0501044 Patent- und Rechtsanwälte Arabellastraße 4 (43) Date of publication of application: 81925 München (DE) 12.09.2007 Bulletin 2007/37 (56) References cited: (73) Proprietor: Richter Gedeon Nyrt. US-A- 4 339 614 1103 Budapest (HU) • MANABU NODE ET.AL.: "Hard acid and soft (72) Inventors: nucleophile system. 2. Demethylation of methyl • VASS, András ethers of alcohol and phenol with an aluminium H-8200 Veszprém (HU) halide-thiolsystem" J. ORG. CHEM., vol. 45, 1980, • DUDÁS, József pages 4275-4277, XP002379759 cited in the H-8200 Veszprém (HU) application Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Healthcare Sterilisation: Challenging Practices, Volume 2
Healthcare Volume 2 Volume Healthcare Sterilisation: Published by Smithers Rapra Technology Ltd, 2014 Challenging Practices Sterilisation: Volume 2 The collection of topics in this second volume of the book reflects challenges the reader to Challenging think beyond standard methods and question why certain current procedures remain static while technological advances abound in other aspects of sterilisation technology. By small means, better practices may come to pass to help answer some of the residual healthcare sterilisation and nosocomial infection queries: • What are some of the current challenges in healthcare sterilisation, and how can they be handled? • What are some of the acceptable current non-traditional sterilisation methods, challenging alternatives, and novel modalities? • What are some of the packaging, validation and statistical considerations of sterilisation Wayne J. Rogers practices? • How does design-of-product and packaging interrelate with sterilisation processing? Practices • Are the current sterility media and practices optimal for recovery of more modified and more resistant viable organism entities and product? • Are there increased sterility and product quality needs with new types of implantables and technological advances within the three dimensional combinations of diagnostics, drug release and challenging medical devices? Wayne J. Rogers Wayne Shawbury, Shrewsbury, Shropshire, SY4 4NR, UK Telephone: +44 (0)1939 250383 Fax: +44 (0)1939 251118 Web: www.polymer-books.com Healthcare Sterilisation: Challenging Practices Volume 2 Wayne J. Rogers A Smithers Group Company Shawbury, Shrewsbury, Shropshire, SY4 4NR, United Kingdom Telephone: +44 (0)1939 250383 Fax: +44 (0)1939 251118 http://www.polymer-books.com First Published in 2014 by Smithers Rapra Technology Ltd Shawbury, Shrewsbury, Shropshire, SY4 4NR, UK ©Smithers Information Ltd., 2014 All rights reserved. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Polar Ozone Depletion
POLAR OZONE DEPLETION Nobel Lecture, December 8, 1995 by MARIO J. MOLINA Department of Earth, Atmospheric and Planetary Sciences, and Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA In On the rigor of Science In that Empire, the Art of Cartography achieved such Perfection that the map of a single Province occupied an entire City, and the map of the Empire an enti- re Province. With time, those unwieldy maps did not satisfy and the Cartographers raised a Map of the Empire, with the size of the Empire and which coincided with it on every point . Jorge Luis Borges INTRODUCTION The ozone layer acts as an atmospheric shield which protects life on Earth against harmful ultraviolet radiation coming from the sun. This shield is fra- gile: in the past two decades it has become very clear that it can be affected by human activities. Roughly 90% of the Earth’s ozone resides in the stratosphere, which is the atmospheric layer characterized by an inverted - that is, increasing - empera- ture profile that rises typically from -210 K at its base at 10-15 km altitude, to roughly 275 K at 50 km altitude. The maximum concentration of ozone is several parts per million; it is continuously being produced in the upper stratosphere by the action of solar radiation on molecular oxygen, and con- tinuously being destroyed by chemical processes involving free radicals. Work by Paul Crutzen in the early 1970’s [1]established that nitrogen oxi- des of natural origin, present at parts per billion levels in the stratosphere, are responsible for most of this chemical destruction, which occurs by means of catalytic cycles. -

Diurnal Variation of Stratospheric Short-Lived Species
THESIS FOR THE DEGREE OF LICENTIATE OF ENGINEERING Diurnal variation of stratospheric short-lived species MARYAM KHOSRAVI Department of Earth and Space Sciences CHALMERS UNIVERSITY OF TECHNOLOGY Gothenburg, Sweden, 2012 Diurnal variation of stratospheric short-lived species MARYAM KHOSRAVI © MARYAM KHOSRAVI, 2012 Technical report No. 52L Department of Earth and Space Sciences Global Environmental Measurements and Modelling Chalmers University of Technology SE-412 96 Gothenburg, Sweden Telephone + 46 (0)31-772 1000 Cover: ClO measurement made by ASUR (Airborne SUbmillimeter Radiometer) on 23 January 2000 in the Arctic stratosphere (top) and corresponding smoothed model simulation (bottom). For detailed information see chapter 4 and paper I. This document was typeset using LATEX. Printed by Chalmers Reproservice Chalmers University of Technology G¨oteborg, Sweden 2012 Diurnal variation of stratospheric short-lived species Maryam Khosravi Chalmers University of Technology Department of Earth and Space Sciences Abstract The depletion of ozone in the stratosphere has a direct impact on the amount of ul- traviolet radiation reaching the Earth’s surface. The ozone abundance and distribution is controlled by the photo-chemical reactions and catalytic cycles involving halogens (chlorine and bromine), odd hydrogen and odd nitrogen species as well as by the at- mospheric transport. An introduction to ozone related chemistry of the stratosphere and modelling of short-lived species using photo-chemical models is presented. A one dimensional (1D) atmospheric model is used in two distinct studies: modeling of short-lived species in the Arctic lower stratosphere (paper I) and in the tropical mid to upper stratosphere (paper II). The first part of this thesis describes the diurnal variation of chlorine monoxide, ClO, which is the most important short-lived species controlling ozone in the polar lower stratosphere during winter and early-spring. -

The Interaction of Hydrogen and Chlorine. the Nature of Photoche?Nical Inhibition
View Article Online / Journal Homepage / Table of Contents for this issue THE INTERACTION OF HYDROGEN AND CHLORINE. 8%5 LX.-The Interaction of Hydrogen and Chlorine. The Nature of Photoche?nical Inhibition. By DAVIDLEONARD CHAPMAN and PATRICKSA~SFIELD MACMAHON. IThas been already remarked (Trans., 1909, 95, 1717) that the gases which are known to retard or prevent the photochemical interaction of hydrogen and chlorine are, without exception, easily reducible substances, and it has been inferred that probably the Published on 01 January 1910. Downloaded by University of Prince Edward Island 25/10/2014 09:30:55. retarding effect is indirectly due to the reduction of the inhibitor by hydrogen or hydrogen chloride. The ground for the above conclusion concerning inhibitors was the following ascertained facts. Oxygen, nitrogen chloride, and the gas formed by the action of moist chlorine or nitric oxide (nitrogen peroxide or nitrosyl chloride) acted as inhibitors, whilst carbon dioxide, nitrogen, and nitrous oxide were mere diluents. That nitrous oxide should appear amongst the list of diluents is not contrary to expectation, since this gas is, in most circumstances, chemically inert. Oxygen is by far the most feeble inhibitor, and it is also the least chemically active." Nitrogen chloride and * As yet, no disappearance of oxygen from an illuminated niixture of hydrogen and chlorine containing it has been detected ; but sufficiently forcible a priori con- siderations can be adduced in support of the assumption that oxygen will slowly react with hydrogen chloride in the presence of chlorine under the influeuce of light. View Article Online 846 CHAPMAN AND MAcMAHON : nitrogen peroxide or nitrosyl chloride are reduced with comparative ease, and they are also powerful inhibitors.