Ep 1831144 B1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SAFETY DATA SHEET Revision Date 28.07.2021 According to Regulation (EC) No
Version 7.0 SAFETY DATA SHEET Revision Date 28.07.2021 according to Regulation (EC) No. 1907/2006 Print Date 02.10.2021 GENERIC EU MSDS - NO COUNTRY SPECIFIC DATA - NO OEL DATA SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifiers Product name : Aluminum iodide Product Number : 208493 Brand : Aldrich REACH No. : A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later registration deadline. CAS-No. : 7784-23-8 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses : Laboratory chemicals, Manufacture of substances 1.3 Details of the supplier of the safety data sheet Company : Sigma-Aldrich Inc. 3050 SPRUCE ST ST. LOUIS MO 63103 UNITED STATES Telephone : +1 314 771-5765 Fax : +1 800 325-5052 1.4 Emergency telephone Emergency Phone # : 800-424-9300 CHEMTREC (USA) +1-703- 527-3887 CHEMTREC (International) 24 Hours/day; 7 Days/week SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 Skin corrosion (Sub-category 1B), H314 Serious eye damage (Category 1), H318 For the full text of the H-Statements mentioned in this Section, see Section 16. 2.2 Label elements Labelling according Regulation (EC) No 1272/2008 Aldrich- 208493 Page 1 of 8 The life science business of Merck operates as MilliporeSigma in the US and Canada Pictogram Signal word Danger Hazard statement(s) H314 Causes severe skin burns and eye damage. -

Determination of Aluminium As Oxide
DETERMINATION OF ALUMINIUM AS OXIDE By William Blum CONTENTS Page I. Introduction 515 II. General principles 516 III. Historical 516 IV. Precipitation of aluminium hydroxide. 518 1. Hydrogen electrode studies 518 (a) The method 518 (b) Apparatus and solutions employed 518 (c) Results of hydrogen electrode experiments 519 (d) Conclusions from hydrogen electrode experiments 520 2. Selection of an indicator for denning the conditions of precipita- '. tion . 522 3. Factors affecting the form of the precipitate 524 4. Precipitation in the presence of iron 525 V. Washing the precipitate . 525 VI. Separation from other elements 526 VII. Ignition and weighing of the precipitate 528 1. Hygroscopicity of aluminium oxide 529 2. Temperature and time of ignition 529 3. Effect of ammonium chloride upon the ignition 531 VIII. Procedure recommended 532 IX. Confirmatory experiments 532 X. Conclusions '534 I. INTRODUCTION Although a considerable number of precipitants have been pro- posed for the determination of aluminium, direct precipitation of aluminium hydroxide by means of ammonium hydroxide, fol- lowed by ignition to oxide, is most commonly used, especially if no separation from iron is desired, in which latter case special methods must be employed. While the general principles involved in this determination are extremely simple, it has long been recog- nized that certain precautions in the precipitation, washing, and ignition are necessary if accurate results are to be obtained. While, however, most of these details have been studied and dis- cussed by numerous authors, it is noteworthy that few publica- tions or textbooks have taken account of all the factors. In the 515 ; 516 Bulletin of the Bureau of Standards [Voi.i3 present paper it seems desirable, therefore, to assemble the various recommendations and to consider their basis and their accuracy. -

Safety Data Sheet
SAFETY DATA SHEET FennoFloc A 19 Ref. /US/EN Revision Date: 02/09/2017 Previous date: 02/09/2017 Print Date:04/20/2017 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING Product information Product name FennoFloc A 19 Recommended use of the chemical and restrictions on use Use of the Substance/Mixture Recommended restrictions on use There are no uses advised against. Supplier's details Kemira Chemicals, Inc. 1000 Parkwood Circle, Suite 500 30339 Atlanta USA Telephone+17704361542, Telefax. +17704363432 HEAD OFFICE Kemira Oyj P.O. Box 330 00101 HELSINKI FINLAND Telephone +358108611 Telefax +358108621124 Emergency telephone number CHEMTREC: 1-800-424-9300 CANUTEC: 1-613-996-6666 2. HAZARDS IDENTIFICATION Classification of the substance or mixture Corrosive to metals; Category 1; May be corrosive to metals.; Serious eye damage; Category 1; Causes serious eye damage.; GHS-Labelling 1/14 SAFETY DATA SHEET FennoFloc A 19 Ref. /US/EN Revision Date: 02/09/2017 Previous date: 02/09/2017 Print Date:04/20/2017 Hazard pictograms : Signal word : Danger Hazard statements : Hazard statements: H290 May be corrosive to metals. H318 Causes serious eye damage. Precautionary statements : Prevention: P234 Keep only in original container. P264 Wash face, hands and any exposed skin thoroughly after handling. P280 Wear protective gloves/ eye protection/ face protection. Response: P390 Absorb spillage to prevent material damage. P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P310 Immediately call a POISON CENTER or doctor/ physician. Storage: P406 Store in corrosive resistant container with a resistant inner liner. -

A Critical Appraisal and Review of Aluminium Chloride Electrolysis for the Production of Aluminium
Bulletin of Eledrochemistry 1 (5) Sep.-Od. 1985, pp. 483488 A CRITICAL APPRAISAL AND REVIEW OF ALUMINIUM CHLORIDE ELECTROLYSIS FOR THE PRODUCTION OF ALUMINIUM C N KANNAN and PS DESIKAN Central Electrochemical Research Institute, Karaikudi - 623 006, ABSTRACT This paper is an attempt to examine the current state of art of aluminium chloride electrolysis through a review of all the available published literature and patents so that this could help the formulation of the plans of work for any serious R&D effort to develop the chloride te&nolcgy in this country. Even though the development of technology for aluminium chloride electrolysis is being carried out in a big way by ALCOA and a few other multinational companies for the past several years, many of the data and information are lacking in published literature and the answers to various critical questions have to be found only through inferences from the meagre information available in patents. It was therefore thought fit to undertake a thorough review of all the basic applied and R&Dwork that are reported in this field and critically assess the various problems to be tackled to evolve a viable technology. This review confines itself to the electrolytic aspect and those relating to the material preparation will be taken up separately later. Key words : Production of aluminium. Molten salt electrolysis, chlorination of alumina INTRODUCTION The most obvious advantages of the aluminium chloride smelting are 131 : he need to develop a new technology for aluminium metal production 1. Substantially lower working temperature (700°C) compared to Hall- T has been felt very much in the major aluminium producing countries Heroult cell (980°C). -

Aluminum Chloride 7446-70-0 >95
SAFETY DATA SHEET Creation Date 10-Sep-2010 Revision Date 27-May-2019 Revision Number 6 1. Identification Product Name Aluminium chloride Cat No. : AC217460000; AC217460025; AC217460050; AC217461000; AC217465000 CAS-No 7446-70-0 Synonyms Aluminium trichloride Recommended Use Laboratory chemicals. Uses advised against Food, drug, pesticide or biocidal product use. Details of the supplier of the safety data sheet Company Fisher Scientific Acros Organics One Reagent Lane One Reagent Lane Fair Lawn, NJ 07410 Fair Lawn, NJ 07410 Tel: (201) 796-7100 Emergency Telephone Number For information US call: 001-800-ACROS-01 / Europe call: +32 14 57 52 11 Emergency Number US:001-201-796-7100 / Europe: +32 14 57 52 99 CHEMTREC Tel. No.US:001-800-424-9300 / Europe:001-703-527-3887 2. Hazard(s) identification Classification This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Skin Corrosion/Irritation Category 1 B Serious Eye Damage/Eye Irritation Category 1 Specific target organ toxicity (single exposure) Category 3 Target Organs - Respiratory system. Label Elements Signal Word Danger Hazard Statements Causes severe skin burns and eye damage May cause respiratory irritation ______________________________________________________________________________________________ Page 1 / 7 Aluminium chloride Revision Date 27-May-2019 ______________________________________________________________________________________________ Precautionary Statements Prevention Do not breathe dust/fume/gas/mist/vapors/spray Wash face, hands and any exposed skin thoroughly after handling Wear protective gloves/protective clothing/eye protection/face protection Use only outdoors or in a well-ventilated area Response Immediately call a POISON CENTER or doctor/physician Inhalation IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing Skin IF ON SKIN (or hair): Take off immediately all contaminated clothing. -
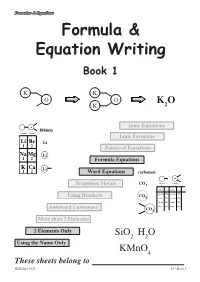
Formula & Equation Writing
Formulae & Equations Formula & Equation Writing Book 1 K K O O K O K 2 Ionic Equations lithium column column 1 2 Ionic Formulae Li Be Li 1 2 Balanced Equations Na Mg Li 1 2 Formula Equations K Ca Li 1 2 Word Equations carbonate valency valency Transition Metals CO3 1 2 name formula name formula ammonium NH4 carbonate CO3 Using Brackets CO 3 cyanide CN chromate CrO4 hydroxide OH sulphate SO4 nitrate NO sulphite SO Awkward Customers 3 3 CO3 More than 2 Elements 2 Elements Only SiO2 H2O Using the Name Only KMnO4 These sheets belong to KHS Sept 2013 page 1 S3 - Book 1 Formulae & Equations What is a Formula ? The formula of a compound tells you two things about the compound :- SiO H O i) which elements are in the compound 2 2 using symbols, and KMnO4 ii) how many atoms of each element are in the compound using numbers. Test Yourself What would be the formula for each of the following? Br H Cl K H C S Al Br O H Cl K H Br Using the Name Only Some compounds have extra information in their carbon monoxide names that allow people to work out and write CO the correct formula. carbon dioxide CO2 The names of the elements appear as usual but dinitrogen tetroxide N O this time the number of each type of atom is 2 4 included using mono- = 1 di- = 12 tri- = 3 tetra- = 4 penta- = 5 hexa- =46 Test Yourself 1 What would be the formula for each of the following? 1. -

United States Patent Office 2 1
2,934,550 Patented Apr. 26, 1960 United States Patent Office 2 1. phenyl or vinyl groups. By “high molecular weight" I mean of molecular weight not less than about 1000. 2,934,550 The material reacted may be in the form of a poly PREPARATION OF CYCLIC METHYLPOLYSILox. siloxane elastomer composition which will, of course, nor ANES BY REACTING ORGANOPOLYSILOXANES mally contain one or more fillers, pigments, etc, or may PHORUSWITH HALDES OF ALUMINUM oR PHOS be in the form of a polysiloxane gum such as is used in the preparation of polysiloxane elastomers. Partially James Jack, Troon, Scotland, assignor to Imperial Chemi cured polysiloxane compositions may also be used and in tionAff." of Great Britain 9 EEE, England, a corpora addition, other high molecular weight linear polysiloxanes 0 may be treated by this process. The advantages of the No Drawing. Application December 8, 1958 process are, however, of most value in the recovery of Serial No. 778,609 elastomersHalides ofand aluminium gums. and phosphorus which may be Claims priority, application Great Britain used in this process include aluminium chloride, alumin January 17, 1958 ium fluoride, aluminium bromide, phosphorus trichlo 5 ride, phosphorus pentachloride and phosphorus oxychlo 8 Claims. (CI. 260-448.2) ride. Aluminium chloride is, however, preferred, since This invention relates to the treatment of high molecu it is relatively inexpensive and does not tend to contami lar weight Substantially linear polysiloxanes and to the nate the products. preparation of low molecular weight cyclic polysiloxanes 20 The process of my invention is normally carried out therefrom. by heating the reactants together in a vessel adapted for High molecular weight linear organopolysiloxanes, for distilation and the low molecular weight cyclic poly example, such as polysiloxane elastomers and gums used siloxanes are distilled off as the reaction proceeds, the for the production thereof, are widely known and used. -

Journal-2019-06
INTELLECTUAL PROPERTY REGISTRY OF SAMOA (IPROS) JOURNAL 2019/06 Publication Date – 4 December 2019 GENERAL INFORMATION The Public is hereby notified that the following persons have applied for the Registration of the Trade Marks as set out in Section 50 of the Intellectual Property Act 2011. Any person who wishes to oppose the registration of any of the under-mentioned Trade Marks, must give notice to the Registrar of Trade Marks, c/- Ministry of Commerce, Industry and Labour, Apia, Samoa within three (3) months from the date of this publication. The notice must be in writing and must include a statement of the grounds of opposition. Each notice of opposition must be accompanied by a filing fee of WST$300. Any person who has any doubts as to whether he/she may be affected by the registration of the Trade Mark in Samoa should consult his/her Solicitor immediately. For any enquiries regarding Trade Marks, please contact the Registry of Intellectual Property at the following telephone number (+685) 20441 or email [email protected] Faafetai, Registrar of Intellectual Property Accepted Trade Marks – Direct Filing (NEWSPAPER VERSION) Application No. : WS/T/7350 Representation of Mark Filing Date : 12 September, 2018 Priority Date : N/A Proprietor/Address : NEST CONSTRUCTION COMPANY LTD Vaitele Uta, Samoa Class(es) 37 Application No. : WS/T/7361 Representation of Mark Filing Date : 24 September, 2018 Priority Date : N/A Proprietor/Address : NIKE INNOVATE C.V. One Bowerman Drive, Beaverton, Oregon 97005--6453, United States of America Class(es) 18 and 25 Application No. : WS/T/7464 Representation of Mark Filing Date : 29 July, 2019 Priority Date : 04/03/2019 Proprietor/Address : REIGN BEVERAGE COMPANY LLC 1547 N. -

(12) Patent Application Publication (10) Pub. No.: US 2015/0175532 A1 Mudduluru Et Al
US 2015O175532A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0175532 A1 Mudduluru et al. (43) Pub. Date: Jun. 25, 2015 (54) PROCESS FOR PREPARATION OF (30) Foreign Application Priority Data 2,3-DIHYDROXY BENZONITRILE Jul. 19, 2012 (IN) ........................... 2926/CHFA2012 (71) Applicant: Laurus Labs Private Limited, Hyderabad (IN) Publication Classification (72) Inventors: Hari Krishna Mudduluru, Hyderabad (51) Int. Cl (IN); Shankar Madhavaram, we Hyderabad (IN) C07C 253/30 (2006.01) (52) U.S. Cl. (73) Assignee: Laurus Labs Private Limited, CPC .................................... C07C 253/30 (2013.01) Hyderabad (IN) (21) Appl. No.: 14/415,527 (57)57 ABSTRACT 1-1. The present invention relates to one pot process for the prepa (22) PCT Filed: Jul.18, 2013 ration of 2,3-dihydroxybenzonitrile from 2,3-dialkoxyben (86). PCT No.: PCT/N2O13AOOO447 Zoic acid without prior isolation of the intermediates. Further the invention relates to the preparation of 2,3-dihydroxyben S371 (c)(1), Zonitrile by dealkylation of 2,3-dialkoxybenzonitrile using (2) Date: Jan. 16, 2015 Suitable aluminum salt-amine complex. US 2015/0175532 A1 Jun. 25, 2015 PROCESS FOR PREPARATION OF 0006. Several reviews have been described for deprotec 2,3-DIHYDROXY BENZONITRILE tion of phenolic ethers. For example, phenolic methyl ethers have deprotected to remove the methyl moiety using hydro PRIORITY gen halide Such as hydrogen chloride or hydrogen bromide under highly acidic conditions; highly dark colored products 0001. This application claims the benefit under Indian Provisional Application No. 2926/CHF/2012 filed 19 Jul. formed in these reactions. In addition the phenolic com 2012 and entitled AN IMPROVED PROCESS FOR THE pounds react further with the halogen compounds used thus PREPARATION OF 2,3-DIHYDROXY BENZONITRILE”, setting a major drawback on this route. -

Chemical Formula of Binary Ionic Compounds – Sheet 1 the Combining Power Or Valency of Silver Is Always 1
Chemical Formula of Binary Ionic Compounds – Sheet 1 The combining power or valency of silver is always 1. All other transition metals are 2 unless otherwise indicated. No. Binary compound Formula No. Binary compound Formula 1 potassium fluoride 26 calcium sulfide 2 calcium chloride 27 lithium bromide 3 barium bromide 28 nickel sulfide 4 silver sulfide 29 zinc phosphide 5 aluminium iodide 30 barium iodide 6 potassium iodide 31 caesium chloride 7 lead(IV) oxide 32 copper bromide 8 zinc nitride 33 sodium nitride 9 silver iodide 34 silver chloride 10 barium fluoride 35 sodium hydride 11 lead(II) iodide 36 potassium nitride 12 silver fluoride 37 cobalt chloride 13 sodium sulfide 38 magnesium sulfide 14 sodium bromide 39 potassium chloride 15 calcium oxide 40 calcium bromide 16 zinc fluoride 41 iron(III) oxide 17 strontium phosphide 42 aluminium fluoride 18 barium sulfide 43 magnesium bromide 19 aluminium oxide 44 iron(III) chloride 20 aluminium chloride 45 barium nitride 21 aluminium sulfide 46 sodium fluoride 22 lead(II) oxide 47 lithium fluoride 23 barium chloride 48 lithium iodide 24 copper chloride 49 lithium hydride 25 barium phosphide 50 potassium oxide “Aluminum” and “cesium” are commonly used alternative spellings for "aluminium" and "caesium that are used in the US. May be freely copied for educational use. ©www.chemicalformula.org Chemical Formula of Binary Ionic Compounds – Sheet 2 The combining power or valency of silver is always 1. All other transition metals are 2 unless otherwise indicated. No. Binary compound Formula No. -

Chemical Resistance Table
CHEMICAL RESISTANCE TABLE Things to consider when choosing a hose • This chemical resistance table indicates if the inner tube of the hose is resistant to specific materials/chemicals throughout different temperatures. • Some materials/chemicals can change color in contact with the hose. If it´s important to have the color unaffected, we recommend you to contact Hydroscand. • For food products the table only indicates whether the inner tube is resistant to the product. This doesn’t automatically significate that the inner tube is approved for food products. • Abrasion, friction and mechanical influence can increase the chemicals aggression and therefore decrease the durability of the hose. • All values in the table are exclusively for the transport of media. • NB! Materials can change color in contact with other kind of materials. • Outer stress is always an important factor to the durability of the hose. NB! This resistance table should be considered as an indication and not a guarantee. International material codes Rubber Plasic NR Natural rubber P.T.F.E. Polytetraflouro ethylene (Teflon®) SBR Styrene butadiene rubber PP Polypropylene NBR Nitrile rubber UPE Ultra high molekular weight EPDM Ethylene-propylene rubber polyethylene IIR Butyl rubber XLPE Cross linked polyethylene (PEX) CR Chloroprene rubber (Neopren) PU Polyurethane CSM Chlorosulfonated polyethylene PE Polyester platsic (Elastomer) rubber (Hypalon) PA Polyamide (Nylon) PVC Polyvinylchloride How to read the table Fitness grade A Good to excelent B Acceptable for limited use -

Title Reaction of 1,2-Dichloroethane with Toluene Author(S) Kunichika, Sango; Oka, Shinzaburo; Sugiyama, Takashi Citation Bullet
Title Reaction of 1,2-Dichloroethane with Toluene Author(s) Kunichika, Sango; Oka, Shinzaburo; Sugiyama, Takashi Bulletin of the Institute for Chemical Research, Kyoto Citation University (1971), 48(6): 276-282 Issue Date 1971-03-24 URL http://hdl.handle.net/2433/76346 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University Bull. Inst. Chem. Res., Kyoto Univ., Vol. 48, No. 6, 1970 Reaction of 1,2-Dichloroethane with Toluene Sango KUNICHIKA, Shinzaburo OKA and Takashi SUGIYAMA* ReceivedDecember 15, 1970 Friedel-Crafts reaction of toluene with 1,2-dichloroethane was studied. The effective catalysts were aluminium bromide, aluminium chloride, gallium chloride and zirconium chloride. Other Lewis acids examined were inactive. The conversion of 1,2-dichloroethane was completed above 50°C and 40% at 25°C with aluminium chloride. Polycondensationproducts were mainly formed via disproportionation of ditolylethanes. Relationship between the isomer distribution and the conver- sion or the reaction time was clarified. INTRODUCTION The reaction of 1,2-dichloroethane with toluene in the presence of Friedel-Crafts type catalyst gives a mixture of six isomers of 1,2-ditolylethane as follows'" : ~~CHCHz OOCHzCHZYJCH3 CH3CH3 CH,J~(%)CH3 CH3 ~~()J) CH3 l) + C1CH,CH,C1+CH,CH,CHzCHz0 CH3SII7 CrCH,CH,0CH3.©CH,CH,CH, -CH,CHt It was described in the previous paper" that these six pure isomers were synthe- sized and an analytical method of these isomers by gaschromatography was established. In the present paper the effective catalysts for this reaction were searched, and the influence of the reaction conditions on the conversion of 1,2-dichloroethane and on the isomer distribution were also examined.