7-N2O4 Tank Cleaning Work Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 7.1 Nitrogen Dioxide
Chapter 7.1 Nitrogen dioxide General description Many chemical species of nitrogen oxides (NOx) exist, but the air pollutant species of most interest from the point of view of human health is nitrogen dioxide (NO2). Nitrogen dioxide is soluble in water, reddish-brown in colour, and a strong oxidant. Nitrogen dioxide is an important atmospheric trace gas, not only because of its health effects but also because (a) it absorbs visible solar radiation and contributes to impaired atmospheric visibility; (b) as an absorber of visible radiation it could have a potential direct role in global climate change if its concentrations were to become high enough; (c) it is, along with nitric oxide (NO), a chief regulator of the oxidizing capacity of the free troposphere by controlling the build-up and fate of radical species, including hydroxyl radicals; and (d) it plays a critical role in determining ozone (O3) concentrations in the troposphere because the photolysis of nitrogen dioxide is the only key initiator of the photochemical formation of ozone, whether in polluted or unpolluted atmospheres (1, 2). Sources On a global scale, emissions of nitrogen oxides from natural sources far outweigh those generated by human activities. Natural sources include intrusion of stratospheric nitrogen oxides, bacterial and volcanic action, and lightning. Because natural emissions are distributed over the entire surface of the earth, however, the resulting background atmospheric concentrations are very small. The major source of anthropogenic emissions of nitrogen oxides into the atmosphere is the combustion of fossil fuels in stationary sources (heating, power generation) and in motor vehicles (internal combustion engines). -

The Formation, Effects and Control of Oxides of Nitrogen in Diesel Engines
International Journal of Applied Engineering Research ISSN 0973-4562 Volume 13, Number 6 (2018) pp. 3200-3209 © Research India Publications. http://www.ripublication.com The Formation, Effects and Control of Oxides of Nitrogen in Diesel Engines Maroa Semakula1and Prof Freddie Inambao2 1,2University of Kwazulu-Natal Durban South-Africa. Abstract HPL High Pressure Loop EGR The transport service industry is a heavy user of diesel IDICI Indirect Injection Compression Ignition propelled engines as prime movers of goods and services. The diesel propelled engine is praised due to its high fuel efficiency, L Length or Piston Stroke reliability and durability. However, the nitrogen emissions as a result of diesel fuel combustion characteristics raise major LPL Low Pressure Loop EGR concerns for the manufacturing industry, environmentalists and health care researchers. The manner in which diesel engines M Organic Residue combust their fuel is the main cause of the nitrogen oxide NG Natural Gas emission proportion. Although there are other sources of nitrogen oxide emission, this work will cover the sources of NO Nitrogen Oxide nitrogen oxides and their formation within the diesel engine, their routes of formation, identify the mechanisms under which NOX Oxides of Nitrogen Excluding Nitrogen Trioxide the formations occur, identify their types and interactions, look Up at the various effects of the oxides of nitrogen on human health and the overall damage to the natural environment, and look OH Water or Hydroxide Radical critically at control systems. -

Nitrogen Oxide - Wikipedia, the Free Encyclopedia Page 1 of 3
Nitrogen oxide - Wikipedia, the free encyclopedia Page 1 of 3 Nitrogen oxide From Wikipedia, the free encyclopedia Nitrogen oxide can refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds: Contents ■ Nitric oxide (NO), nitrogen(II) oxide ■ Nitrogen dioxide (NO2), nitrogen(IV) oxide ■ 1 NOx ■ Nitrous oxide (N2O), nitrogen(I) oxide ■ 2 Derivatives ■ Nitrate radical (NO3), nitrogen(VI) oxide ■ 3 See also ■ Dinitrogen trioxide (N2O3), nitrogen(II,IV) oxide ■ 4 References ■ Dinitrogen tetroxide (N2O4), nitrogen(IV) oxide ■ Dinitrogen pentoxide (N2O5), nitrogen(V) oxide In atmospheric chemistry and air pollution and related fields, nitrogen oxides refers specifically to NOx [1][2] (NO and NO2). Only the first three of these compounds can be isolated at room temperature. N2O3, N2O4, and N2O5 all decompose rapidly at room temperature. Nitrate radical is very reactive. N2O is stable and rather unreactive at room temperature, while NO and NO2 are quite reactive but nevertheless quite stable when isolated. Dinitrogen trioxide, Nitric oxide, NO Nitrogen dioxide, NO2 Nitrous oxide, N2O N2O3 Dinitrogen tetroxide, Dinitrogen pentoxide, N2O4 N2O5 http://en.wikipedia.org/wiki/Nitrogen_oxide 11/2/2010 Nitrogen oxide - Wikipedia, the free encyclopedia Page 2 of 3 NOx Main article: NOx NOx (often written NOx) refers to NO and NO2. They are produced during combustion, especially at high temperature. These two chemicals are important trace species in Earth's atmosphere. In the troposphere, during daylight, NO reacts with partly oxidized organic species (or the peroxy radical) to form NO2, which is then photolyzed by sunlight to reform NO: NO + CH3O2 → NO2 + CH3O NO2 + sunlight → NO + O The oxygen atom formed in the second reaction then goes on to form ozone; this series of reactions is the main source of tropospheric ozone. -

Nitrogen Oxides
Pollution Prevention and Abatement Handbook WORLD BANK GROUP Effective July 1998 Nitrogen Oxides Nitrogen oxides (NOx) in the ambient air consist 1994). The United States generates about 20 mil- primarily of nitric oxide (NO) and nitrogen di- lion metric tons of nitrogen oxides per year, about oxide (NO2). These two forms of gaseous nitro- 40% of which is emitted from mobile sources. Of gen oxides are significant pollutants of the lower the 11 million to 12 million metric tons of nitrogen atmosphere. Another form, nitrous oxide (N2O), oxides that originate from stationary sources, is a greenhouse gas. At the point of discharge about 30% is the result of fuel combustion in large from man-made sources, nitric oxide, a colorless, industrial furnaces and 70% is from electric utility tasteless gas, is the predominant form of nitro- furnaces (Cooper and Alley 1986). gen oxide. Nitric oxide is readily converted to the much more harmful nitrogen dioxide by Occurrence in Air and Routes of Exposure chemical reaction with ozone present in the at- mosphere. Nitrogen dioxide is a yellowish-or- Annual mean concentrations of nitrogen dioxide ange to reddish-brown gas with a pungent, in urban areas throughout the world are in the irritating odor, and it is a strong oxidant. A por- range of 20–90 micrograms per cubic meter (µg/ tion of nitrogen dioxide in the atmosphere is con- m3). Maximum half-hour values and maximum 24- verted to nitric acid (HNO3) and ammonium hour values of nitrogen dioxide can approach 850 salts. Nitrate aerosol (acid aerosol) is removed µg/m3 and 400 µg/m3, respectively. -
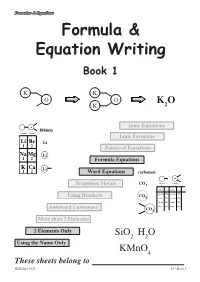
Formula & Equation Writing
Formulae & Equations Formula & Equation Writing Book 1 K K O O K O K 2 Ionic Equations lithium column column 1 2 Ionic Formulae Li Be Li 1 2 Balanced Equations Na Mg Li 1 2 Formula Equations K Ca Li 1 2 Word Equations carbonate valency valency Transition Metals CO3 1 2 name formula name formula ammonium NH4 carbonate CO3 Using Brackets CO 3 cyanide CN chromate CrO4 hydroxide OH sulphate SO4 nitrate NO sulphite SO Awkward Customers 3 3 CO3 More than 2 Elements 2 Elements Only SiO2 H2O Using the Name Only KMnO4 These sheets belong to KHS Sept 2013 page 1 S3 - Book 1 Formulae & Equations What is a Formula ? The formula of a compound tells you two things about the compound :- SiO H O i) which elements are in the compound 2 2 using symbols, and KMnO4 ii) how many atoms of each element are in the compound using numbers. Test Yourself What would be the formula for each of the following? Br H Cl K H C S Al Br O H Cl K H Br Using the Name Only Some compounds have extra information in their carbon monoxide names that allow people to work out and write CO the correct formula. carbon dioxide CO2 The names of the elements appear as usual but dinitrogen tetroxide N O this time the number of each type of atom is 2 4 included using mono- = 1 di- = 12 tri- = 3 tetra- = 4 penta- = 5 hexa- =46 Test Yourself 1 What would be the formula for each of the following? 1. -

Aqueous Catalytic Disproportionation and Oxidation of Nitric Oxide
Environ. Sci. Technol. 1998, 32, 876-881 Noncatalytic oxidation/reduction and absorption tech- Aqueous Catalytic niques (chemical scrubbing) have the advantage of being able to eliminate both NOx and SOx simultaneously (5, 6). Disproportionation and Oxidation of The primary impediment to their industrial application has Nitric Oxide been the low solubility of NO in aqueous solution. Because nitric oxide is 90-95% of the NOx present in typical flue gas streams (7), it has been necessary to preoxidize NO to NO2 JOSEPH H. MACNEIL, before the scrubber. Direct oxidation with O occurs slowly POLLY A. BERSETH, 2 at the low NO concentrations present in exhaust streams. GLENN WESTWOOD, AND The additional complexity and expense of using alternative WILLIAM C. TROGLER* - oxidants, such as OCl and H2O2, have prevented widespread Department of Chemistry and Biochemistry, University of use of this method. Other approaches to treating NO in California at San Diego, La Jolla, California 92093-0358 aqueous scrubbers have included the addition of heavy metal chelators to sequester nitric oxide for subsequent removal (8) and even the addition of yellow phosphorus emulsions Nitric oxide, a byproduct of combustion exhaust, is a key and O2 to oxidize nitric oxide to a combination of nitrite and nitrate salts (9). species that leads to urban photochemical smog. Nitric oxide exhibits low aqueous solubility, and it has proved In this paper we report a novel catalytic approach for difficult to remove NO from gas streams by aqueous scrubbing removing NO in aqueous solution with supported palladium or platinum metal. The catalysts display activity at ambient methods. -

Chapter 10 Acronyms.Pdf
10. Acronyms °C Degrees Celsius (Centigrade) µm Micrometer (micron) 1D One dimensional 3D Three dimensional 3GAHHS Third-generation advanced high-strength steels A Ampere A/C Air conditioning ABR Advanced Battery Research AC Alternating current ACE Advanced Combustion Engines (VTO program) ACEC Advanced Combustion and Emissions Control ACI Advanced compression ignition ADAS Advanced Driver Assistance Systems ADP Advanced drying process AEC Advanced engine combustion AEC Automotive Electronics Council AEV All-electric vehicle AFDC Alternative Fuels Data Center AFV Alternative fuel vehicle Ag Silver Ah Ampere-hour AHHS Advanced high-strength steels AIM Accelerated insertion of materials AKI Anti-knock index Al Aluminum ALD Atomic layer deposition AlF3 Aluminum fluoride AlNiCo Aluminum-nickel-cobalt AMR Annual Merit Review AMT Air maintenance technology AMT Automated manual transmission ANL Argonne National Laboratory APEEM Advanced Power Electronics and Electric Motors API American Petroleum Institute API Application programming interface APS Advanced photon source APTA American Public Transportation Association ARA Automotive Recyclers Association ARC Affordable Rankine cycle ARK Abuse reaction kinetics ARL Army Research Laboratory Acronyms 10-1 2016 ANNUAL MERIT REVIEW, VEHICLE TECHNOLOGIES OFFICE ARPA-E Advanced Research Projects Agency - Energy ASCR Advanced scientific computing research ASI Area specific impedance ASSERT Analysis of Sustainability, Scale, Economics, Risk, and Trade ASTM American Society for Testing and Materials ATF -

Kinetics of Nitrogen Oxide Reduction by Means of Ammonia on a Polish Carbon Sorbent
Polish Journal of Environmental Studies Vol. 11, No. 6 (2002), 689-693 Kinetics of Nitrogen Oxide Reduction by Means of Ammonia on a Polish Carbon Sorbent K. Jastrz¹b*, J. Szarawara aculty of Chemistry, Silesian University of Technology, 44-100 Gliwice, B. Krzywoustego Str. 6, Poland, Received: 16 January, 2002 Accepted: 13 May, 2002 Abstract This paper studies nitrogen oxide reduction by means of ammonia in a flow reactor with a fixed bed of the Polish carbon sorbent AKP-5. The kinetic curves â(t), showing the dependence of the degree of reduction of nitrogen oxide on time have been determined for four inlet NO concentrations. On the basis of the degree of NO reduction determined for the steady state conditions, a simplified model of the kinetic equation has been developed and verified. The results obtained are very important for environmental protection. Keywords: nitrogen oxides, carbon sorbent, selective catalytic reduction, kinetics of reaction Introduction of reactants), the physical adsorption and chemisorption of the substrates on the surface, and a series of catalytic surface The process based on the use of specially prepared reactions. Moreover, the process occurs according to carbon sorbents (so-called active cokes) is an interesting a complex chemical mechanism [7]. In the case of the SCR modification of the selective catalytic reduction (SCR) process, the reduction of NO by ammonia is most often x method. In this process the sorbent plays a double role: described by the following overall equation: it is both an adsorbent of nitrogen oxides and a classical catalyst of NO reduction. An advantage of this method is 4 NO + 4 NH + O = 4 N + 6 H O (1) x 3 2 2 2 the low temperature of the process (100 ÷ 170°C). -

Nitrogen Oxide Emissions from Air Traffic
TECHNOLOGIE 369 CHIMIA 44 (1990) Nr. II (November) Chimia 44 (1990) 369-371 ~'1Schwei=. Chemiker-Verband; ISSN 0009-4293 Nitrogen Oxide Emissions from Air Traffic Robert A. Egli* Rober! A. Eg/i: Born 1926 in Rheineck, Switzer- land. After completing his chemistry education at the HTL Winterthur (1948), he worked for 10years as a group leader in an organic synthesis laboratory for Ci/og AG in Schaffhausen, later as head of the Ahstract. About 153 million metric tons of aviation fuel were consumed in 1987, which analytical development laboratory, and in addition was ca. ]3% of the world's consumption of transportation fuel. Burning this fuel to this job. for 13 years quality control director of Ci/ag AG. From 1985 to 1989, he was responsible produced ca. 2.75 million tons of nitrogen oxides, calculated as N0 , using an average 2 for patents, library, and literature searches. He emission index £1 of 18 g N02 per kg fuel. 0.92 million tons of N02 was exhausted published 17 papers, mainly in the field of organic between 9 and 13 km, which is an especially endangered altitude range, estimated with an analytical chemistry. Since January 1990, Egli is £f of 15 g N02 per kg fuel. Air traffic is the main NO, source between 9 and 13 km. Since working as an independent consultant. For many the NO, background concentration at this altitude is low and the possible lifetime of an years, his special interests have been air pollution problems and climatology. admixture two orders of magnitude larger compared to the ground, these NO .•emissions can lead to an important increase of tropospheric ozone, which contributes to the g]obal greenhouse warming. -

Nitric Acid Plants (PDF)
Office of Air and Radiation December 2010 AVAILABLE AND EMERGING TECHNOLOGIES FOR REDUCING GREENHOUSE GAS EMISSIONS FROM THE NITRIC ACID PRODUCTION INDUSTRY Available and Emerging Technologies for Reducing Greenhouse Gas Emissions from the Nitric Acid Production Industry Prepared by the Sector Policies and Programs Division Office of Air Quality Planning and Standards U.S. Environmental Protection Agency Research Triangle Park, North Carolina 27711 December 2010 Table of Contents Abbreviations and Acronyms p.1 I Introduction /Purpose of this Document p.2 II. Description of the Nitric Acid Production Process p.3 A. Weak Nitric Acid Production p.3 B. High-Strength Nitric Acid Production p.6 III. N2O Emissions and Nitric Acid Production Process p. 7 IV. Summary of Control Measures p.9 A. Primary Controls p.10 B. Secondary Control p.11 C. Tertiary Controls p.13 D. Selective Catalytic Reduction p.16 V. Other Greenhouse Gas Emissions p.16 VI. Energy Efficiency Improvements p.17 VII. EPA Contacts p.19 VIII. References p.20 Appendix A – Southeast Idaho Energy p.23 Appendix B - US Nitric Acid Plants p.24 Appendix C – CDM Monitoring Reports p.25 Abbreviations and Acronyms atm Pressure in atmospheres BACT Best Available Control Technologies Btu British Thermal Unit Btu/lb Btu per lb of 100% nitric acid CAR Climate Action Reserve CDM Clean Development Mechanism CHP Combined Heat and Power CO2 Carbon dioxide CO2e Carbon dioxide equivalent EU European Union GHG Greenhouse Gases H2 Hydrogen IPCC Intergovernmental Panel on Climate Change IPPC Industrial Pollution Prevention and Control JI Joint Implementation kg N2O/tonne kilograms of N2O per tonne of 100% nitric acid kg CO2e/tonne kilograms of CO2e per tonne of 100% nitric acid lb N2O/ton pounds of N2O per ton of 100% nitric acid N2 Nitrogen NH3 Ammonia NO Nitric oxide NO2 Nitrogen dioxide NOx Nitrogen oxides NSCR Nonselective Catalytic Reduction N2 O4 Nitrogen tetraoxide N2O Nitrous oxide O2 Oxygen PSD Prevention of Significant Deterioration SCR Selective Catalytic Reduction TPD Tons per day 1 I. -
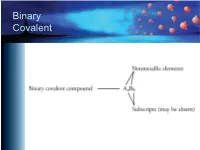
Binary Covalent Common Names
Binary Covalent Common Names – H2O, water – NH3, ammonia – CH4, methane – C2H6, ethane – C3H8, propane – C4H10, butane – C5H12, pentane – C6H14, hexane Naming Binary Covalent CompounDs • If the subscript for the first element is greater than one, inDicate the subscript with a prefix. – We Do not write mono- on the first name. – Leave the "a" off the enD of the prefixes that enD in "a" anD the “o” off of mono- if they are placeD in front of an element that begins with a vowel (oxygen or ioDine). Prefixes mon(o) hex(a) di hept(a) tri oct(a) tetr(a) non(a) pent(a) dec(a) Nitrogen OxiDe Names • N2O3 – name starts with di • N2O5 – name starts with di • NO2 – no initial prefix • NO – no initial prefix Naming Binary Covalent CompounDs • Follow the prefix with the name of the first element in the formula. – N2O3 – dinitrogen – N2O3 – dinitrogen – NO2 – nitrogen – NO – nitrogen Naming Binary Covalent CompounDs • Write a prefix to inDicate the subscript for the seconD element. (Remember to leave the “o” off of mono- anD the “a” off of the prefixes that enD in “a” when they are placeD in front of a name that begins with a vowel.) – N2O3 – dinitrogen tri – N2O5 – dinitrogen pent – NO2 – nitrogen di – NO – nitrogen mon Naming Binary Covalent CompounDs • Write the root of the name of the seconD symbol in the formula. (See the next sliDe.) – N2O3 – dinitrogen triox – N2O5 – dinitrogen pentox – NO2 – nitrogen diox – NO – nitrogen monox Roots of Nonmetals H hydr- F fluor- C carb- Cl chlor- N nitr- Br brom- P phosph- I iod- O ox- S sulf- Se selen- Naming Binary Covalent CompounDs • AdD -ide to the enD of the name. -

Modeling the Vapor – Liquid Equilibrium and Association
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Open Archive Toulouse Archive Ouverte MODELING THE VAPOR – LIQUID EQUILIBRIUM AND ASSOCIATION OF NITROGEN DIOXIDE / DINITROGEN TETROXIDE AND ITS MIXTURES WITH CARBON DIOXIDE A. Belkadi 1,2 , F. Llovell 3, V. Gerbaud 1*, L. F. Vega 2,3*,+ 1Université de Toulouse, LGC (Laboratoire de Génie Chimique), CNRS, INP, UPS, 5 rue Paulin Talabot, F-31106 Toulouse Cedex 01 – France 2MATGAS Research Center, Campus UAB. 08193 Bellaterra, Barcelona Spain 3Institut de Ciència de Materials de Barcelona, (ICMAB-CSIC), Consejo Superior de Investigaciones Científicas, Campus de la UAB, 08193 Bellaterra, Barcelona, Spain * Corresponding authors: [email protected], [email protected]. +Present address: Carburos Metálicos-Grup Air Products. C/ Aragón 300. 08009 Barcelona. Spain. 1 Abstract We have used in this work the crossover soft-SAFT equation of state to model nitrogen dioxide/dinitrogen tetraoxide (NO 2/N 2O4), carbon dioxide (CO 2) and their mixtures. The prediction of the vapor – liquid equilibrium of this mixture is of utmost importance to correctly assess the NO 2 monomer amount that is the oxidizing agent of vegetal macromolecules in the CO 2 + NO 2 / N 2O4 reacting medium under supercritical conditions. The quadrupolar effect was explicitly considered when modeling carbon dioxide, enabling to obtain an excellent description of the vapor-liquid equilibria diagrams. NO 2 was modeled as a self associating molecule with a single association site to account for the strong associating character of the NO 2 molecule. Again, the vapor- liquid equilibrium of NO 2 was correctly modeled.