Nitrogen Oxide - Wikipedia, the Free Encyclopedia Page 1 of 3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 7.1 Nitrogen Dioxide
Chapter 7.1 Nitrogen dioxide General description Many chemical species of nitrogen oxides (NOx) exist, but the air pollutant species of most interest from the point of view of human health is nitrogen dioxide (NO2). Nitrogen dioxide is soluble in water, reddish-brown in colour, and a strong oxidant. Nitrogen dioxide is an important atmospheric trace gas, not only because of its health effects but also because (a) it absorbs visible solar radiation and contributes to impaired atmospheric visibility; (b) as an absorber of visible radiation it could have a potential direct role in global climate change if its concentrations were to become high enough; (c) it is, along with nitric oxide (NO), a chief regulator of the oxidizing capacity of the free troposphere by controlling the build-up and fate of radical species, including hydroxyl radicals; and (d) it plays a critical role in determining ozone (O3) concentrations in the troposphere because the photolysis of nitrogen dioxide is the only key initiator of the photochemical formation of ozone, whether in polluted or unpolluted atmospheres (1, 2). Sources On a global scale, emissions of nitrogen oxides from natural sources far outweigh those generated by human activities. Natural sources include intrusion of stratospheric nitrogen oxides, bacterial and volcanic action, and lightning. Because natural emissions are distributed over the entire surface of the earth, however, the resulting background atmospheric concentrations are very small. The major source of anthropogenic emissions of nitrogen oxides into the atmosphere is the combustion of fossil fuels in stationary sources (heating, power generation) and in motor vehicles (internal combustion engines). -

The Formation, Effects and Control of Oxides of Nitrogen in Diesel Engines
International Journal of Applied Engineering Research ISSN 0973-4562 Volume 13, Number 6 (2018) pp. 3200-3209 © Research India Publications. http://www.ripublication.com The Formation, Effects and Control of Oxides of Nitrogen in Diesel Engines Maroa Semakula1and Prof Freddie Inambao2 1,2University of Kwazulu-Natal Durban South-Africa. Abstract HPL High Pressure Loop EGR The transport service industry is a heavy user of diesel IDICI Indirect Injection Compression Ignition propelled engines as prime movers of goods and services. The diesel propelled engine is praised due to its high fuel efficiency, L Length or Piston Stroke reliability and durability. However, the nitrogen emissions as a result of diesel fuel combustion characteristics raise major LPL Low Pressure Loop EGR concerns for the manufacturing industry, environmentalists and health care researchers. The manner in which diesel engines M Organic Residue combust their fuel is the main cause of the nitrogen oxide NG Natural Gas emission proportion. Although there are other sources of nitrogen oxide emission, this work will cover the sources of NO Nitrogen Oxide nitrogen oxides and their formation within the diesel engine, their routes of formation, identify the mechanisms under which NOX Oxides of Nitrogen Excluding Nitrogen Trioxide the formations occur, identify their types and interactions, look Up at the various effects of the oxides of nitrogen on human health and the overall damage to the natural environment, and look OH Water or Hydroxide Radical critically at control systems. -

Nitrogen Oxides
Pollution Prevention and Abatement Handbook WORLD BANK GROUP Effective July 1998 Nitrogen Oxides Nitrogen oxides (NOx) in the ambient air consist 1994). The United States generates about 20 mil- primarily of nitric oxide (NO) and nitrogen di- lion metric tons of nitrogen oxides per year, about oxide (NO2). These two forms of gaseous nitro- 40% of which is emitted from mobile sources. Of gen oxides are significant pollutants of the lower the 11 million to 12 million metric tons of nitrogen atmosphere. Another form, nitrous oxide (N2O), oxides that originate from stationary sources, is a greenhouse gas. At the point of discharge about 30% is the result of fuel combustion in large from man-made sources, nitric oxide, a colorless, industrial furnaces and 70% is from electric utility tasteless gas, is the predominant form of nitro- furnaces (Cooper and Alley 1986). gen oxide. Nitric oxide is readily converted to the much more harmful nitrogen dioxide by Occurrence in Air and Routes of Exposure chemical reaction with ozone present in the at- mosphere. Nitrogen dioxide is a yellowish-or- Annual mean concentrations of nitrogen dioxide ange to reddish-brown gas with a pungent, in urban areas throughout the world are in the irritating odor, and it is a strong oxidant. A por- range of 20–90 micrograms per cubic meter (µg/ tion of nitrogen dioxide in the atmosphere is con- m3). Maximum half-hour values and maximum 24- verted to nitric acid (HNO3) and ammonium hour values of nitrogen dioxide can approach 850 salts. Nitrate aerosol (acid aerosol) is removed µg/m3 and 400 µg/m3, respectively. -

Aqueous Catalytic Disproportionation and Oxidation of Nitric Oxide
Environ. Sci. Technol. 1998, 32, 876-881 Noncatalytic oxidation/reduction and absorption tech- Aqueous Catalytic niques (chemical scrubbing) have the advantage of being able to eliminate both NOx and SOx simultaneously (5, 6). Disproportionation and Oxidation of The primary impediment to their industrial application has Nitric Oxide been the low solubility of NO in aqueous solution. Because nitric oxide is 90-95% of the NOx present in typical flue gas streams (7), it has been necessary to preoxidize NO to NO2 JOSEPH H. MACNEIL, before the scrubber. Direct oxidation with O occurs slowly POLLY A. BERSETH, 2 at the low NO concentrations present in exhaust streams. GLENN WESTWOOD, AND The additional complexity and expense of using alternative WILLIAM C. TROGLER* - oxidants, such as OCl and H2O2, have prevented widespread Department of Chemistry and Biochemistry, University of use of this method. Other approaches to treating NO in California at San Diego, La Jolla, California 92093-0358 aqueous scrubbers have included the addition of heavy metal chelators to sequester nitric oxide for subsequent removal (8) and even the addition of yellow phosphorus emulsions Nitric oxide, a byproduct of combustion exhaust, is a key and O2 to oxidize nitric oxide to a combination of nitrite and nitrate salts (9). species that leads to urban photochemical smog. Nitric oxide exhibits low aqueous solubility, and it has proved In this paper we report a novel catalytic approach for difficult to remove NO from gas streams by aqueous scrubbing removing NO in aqueous solution with supported palladium or platinum metal. The catalysts display activity at ambient methods. -

Chapter 10 Acronyms.Pdf
10. Acronyms °C Degrees Celsius (Centigrade) µm Micrometer (micron) 1D One dimensional 3D Three dimensional 3GAHHS Third-generation advanced high-strength steels A Ampere A/C Air conditioning ABR Advanced Battery Research AC Alternating current ACE Advanced Combustion Engines (VTO program) ACEC Advanced Combustion and Emissions Control ACI Advanced compression ignition ADAS Advanced Driver Assistance Systems ADP Advanced drying process AEC Advanced engine combustion AEC Automotive Electronics Council AEV All-electric vehicle AFDC Alternative Fuels Data Center AFV Alternative fuel vehicle Ag Silver Ah Ampere-hour AHHS Advanced high-strength steels AIM Accelerated insertion of materials AKI Anti-knock index Al Aluminum ALD Atomic layer deposition AlF3 Aluminum fluoride AlNiCo Aluminum-nickel-cobalt AMR Annual Merit Review AMT Air maintenance technology AMT Automated manual transmission ANL Argonne National Laboratory APEEM Advanced Power Electronics and Electric Motors API American Petroleum Institute API Application programming interface APS Advanced photon source APTA American Public Transportation Association ARA Automotive Recyclers Association ARC Affordable Rankine cycle ARK Abuse reaction kinetics ARL Army Research Laboratory Acronyms 10-1 2016 ANNUAL MERIT REVIEW, VEHICLE TECHNOLOGIES OFFICE ARPA-E Advanced Research Projects Agency - Energy ASCR Advanced scientific computing research ASI Area specific impedance ASSERT Analysis of Sustainability, Scale, Economics, Risk, and Trade ASTM American Society for Testing and Materials ATF -

Kinetics of Nitrogen Oxide Reduction by Means of Ammonia on a Polish Carbon Sorbent
Polish Journal of Environmental Studies Vol. 11, No. 6 (2002), 689-693 Kinetics of Nitrogen Oxide Reduction by Means of Ammonia on a Polish Carbon Sorbent K. Jastrz¹b*, J. Szarawara aculty of Chemistry, Silesian University of Technology, 44-100 Gliwice, B. Krzywoustego Str. 6, Poland, Received: 16 January, 2002 Accepted: 13 May, 2002 Abstract This paper studies nitrogen oxide reduction by means of ammonia in a flow reactor with a fixed bed of the Polish carbon sorbent AKP-5. The kinetic curves â(t), showing the dependence of the degree of reduction of nitrogen oxide on time have been determined for four inlet NO concentrations. On the basis of the degree of NO reduction determined for the steady state conditions, a simplified model of the kinetic equation has been developed and verified. The results obtained are very important for environmental protection. Keywords: nitrogen oxides, carbon sorbent, selective catalytic reduction, kinetics of reaction Introduction of reactants), the physical adsorption and chemisorption of the substrates on the surface, and a series of catalytic surface The process based on the use of specially prepared reactions. Moreover, the process occurs according to carbon sorbents (so-called active cokes) is an interesting a complex chemical mechanism [7]. In the case of the SCR modification of the selective catalytic reduction (SCR) process, the reduction of NO by ammonia is most often x method. In this process the sorbent plays a double role: described by the following overall equation: it is both an adsorbent of nitrogen oxides and a classical catalyst of NO reduction. An advantage of this method is 4 NO + 4 NH + O = 4 N + 6 H O (1) x 3 2 2 2 the low temperature of the process (100 ÷ 170°C). -

Nitrogen Oxide Emissions from Air Traffic
TECHNOLOGIE 369 CHIMIA 44 (1990) Nr. II (November) Chimia 44 (1990) 369-371 ~'1Schwei=. Chemiker-Verband; ISSN 0009-4293 Nitrogen Oxide Emissions from Air Traffic Robert A. Egli* Rober! A. Eg/i: Born 1926 in Rheineck, Switzer- land. After completing his chemistry education at the HTL Winterthur (1948), he worked for 10years as a group leader in an organic synthesis laboratory for Ci/og AG in Schaffhausen, later as head of the Ahstract. About 153 million metric tons of aviation fuel were consumed in 1987, which analytical development laboratory, and in addition was ca. ]3% of the world's consumption of transportation fuel. Burning this fuel to this job. for 13 years quality control director of Ci/ag AG. From 1985 to 1989, he was responsible produced ca. 2.75 million tons of nitrogen oxides, calculated as N0 , using an average 2 for patents, library, and literature searches. He emission index £1 of 18 g N02 per kg fuel. 0.92 million tons of N02 was exhausted published 17 papers, mainly in the field of organic between 9 and 13 km, which is an especially endangered altitude range, estimated with an analytical chemistry. Since January 1990, Egli is £f of 15 g N02 per kg fuel. Air traffic is the main NO, source between 9 and 13 km. Since working as an independent consultant. For many the NO, background concentration at this altitude is low and the possible lifetime of an years, his special interests have been air pollution problems and climatology. admixture two orders of magnitude larger compared to the ground, these NO .•emissions can lead to an important increase of tropospheric ozone, which contributes to the g]obal greenhouse warming. -

COVALENT COMPOUNDS Unit 2 COVALENT BOND
COVALENT COMPOUNDS Unit 2 COVALENT BOND Forms between two non-metals! Electrons are shared! Co= share Valent = electrons Since electrons are shared, no need to balance charges Covalent bonds form molecules. Molecule - group of neutral atoms held together with a covalent bond Diatomic molecules – molecule consisting of 2 atoms MOLECULES H2O2Br2F2I2N2Cl2 Compound composed of molecules is a molecular compound. MOLECULAR COMPOUNDS Molecular Compounds Properties Gases or liquids Low melting/boiling points Usually made from 2 nonmetals bonded covalently Do not conduct electricity (solid or dissolved) Molecular chemical formula shows how many atoms of each element a molecule contains. H2O – Molecular formula of water 2 Hydrogen atoms, 1 Oxygen atom CO2 – Molecular formula for Carbon Dioxide MOLECULAR 1 Carbon atom, 2 Oxygen atoms FORMULAS H:H - Lewis Dot H-H – Structural Formula H2 – Molecular chemical formula LEWIS STRUCTURES COVALENT BONDING To draw a Lewis Structure, one must know the types of atoms in the molecule, the number of atoms in the molecule and the number of valence electrons for each atom in the molecule. DOUBLE & TRIPLE COVALENT BONDS Sharing a pair of electrons creates a single covalent bond Sharing more than one pair of electrons creates double and triple covalent bonds. 2 shared pairs – Double covalent bond 3 shared pairs – Triple covalent bond Carbon, Nitrogen, Oxygen 1. First element is named first, using the full name of the element 2. The second element is named by dropping ending and adding RULES FOR “–ide” NAMING 3. Prefixes are used to denote # COVALENT of each atom present COMPOUNDS 4. Prefix mono- is never used on the first element 5. -
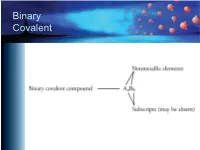
Binary Covalent Common Names
Binary Covalent Common Names – H2O, water – NH3, ammonia – CH4, methane – C2H6, ethane – C3H8, propane – C4H10, butane – C5H12, pentane – C6H14, hexane Naming Binary Covalent CompounDs • If the subscript for the first element is greater than one, inDicate the subscript with a prefix. – We Do not write mono- on the first name. – Leave the "a" off the enD of the prefixes that enD in "a" anD the “o” off of mono- if they are placeD in front of an element that begins with a vowel (oxygen or ioDine). Prefixes mon(o) hex(a) di hept(a) tri oct(a) tetr(a) non(a) pent(a) dec(a) Nitrogen OxiDe Names • N2O3 – name starts with di • N2O5 – name starts with di • NO2 – no initial prefix • NO – no initial prefix Naming Binary Covalent CompounDs • Follow the prefix with the name of the first element in the formula. – N2O3 – dinitrogen – N2O3 – dinitrogen – NO2 – nitrogen – NO – nitrogen Naming Binary Covalent CompounDs • Write a prefix to inDicate the subscript for the seconD element. (Remember to leave the “o” off of mono- anD the “a” off of the prefixes that enD in “a” when they are placeD in front of a name that begins with a vowel.) – N2O3 – dinitrogen tri – N2O5 – dinitrogen pent – NO2 – nitrogen di – NO – nitrogen mon Naming Binary Covalent CompounDs • Write the root of the name of the seconD symbol in the formula. (See the next sliDe.) – N2O3 – dinitrogen triox – N2O5 – dinitrogen pentox – NO2 – nitrogen diox – NO – nitrogen monox Roots of Nonmetals H hydr- F fluor- C carb- Cl chlor- N nitr- Br brom- P phosph- I iod- O ox- S sulf- Se selen- Naming Binary Covalent CompounDs • AdD -ide to the enD of the name. -

Enrichment of Denitrifying Bacterial Community Using Nitrite As an Electron Acceptor for Nitrogen Removal from Wastewater
water Article Enrichment of Denitrifying Bacterial Community Using Nitrite as an Electron Acceptor for Nitrogen Removal from Wastewater Renda Yao, Quan Yuan and Kaijun Wang * State Key Joint Laboratory of Environment Simulation and Pollution Control, School of Environment, Tsinghua University, Beijing 100084, China; [email protected] (R.Y.); [email protected] (Q.Y.) * Correspondence: [email protected]; Tel.: +86-10-6278-9411 Received: 31 October 2019; Accepted: 17 December 2019; Published: 20 December 2019 Abstract: This work aimed to enrich a denitrifying bacterial community for economical denitrification via nitrite to provide the basic objects for enhancing nitrogen removal from wastewater. A sequencing batch reactor (SBR) with continuous nitrite and acetate feeding was operated by reasonably adjusting the supply rate based on the reaction rate, and at a temperature of 20 2 C, pH of 7.5 0.2, ± ◦ ± and dissolved oxygen (DO) of 0 mg/L. The results revealed that the expected nitrite concentration can be achieved during the whole anoxic reaction period. The nitrite denitrification rate of nitrogen removal from synthetic wastewater gradually increased from approximately 10 mg/(L h) to 275.35 mg/(L h) over 12 days (the specific rate increased from 3.83 mg/(g h) to 51.80 mg/(g h)). Correspondingly, the chemical oxygen demand/nitrogen (COD/N) ratio of reaction decreased from 7.9 to 2.7. Both nitrite and nitrate can be used as electron acceptors for denitrification. The mechanism of this operational mode was determined via material balance analysis of substrates in a typical cycle. High-throughput sequencing showed that the main bacterial community was related to denitrification, which accounted for 84.26% in the cultivated sludge, and was significantly higher than the 2.16% in the seed sludge. -

Nitrate Formation from Heterogeneous Uptake of Dinitrogen Pentoxide During a Severe Winter Haze in Southern China” by Hui Yun Et Al
Atmos. Chem. Phys. Discuss., https://doi.org/10.5194/acp-2018-698-RC1, 2018 © Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Interactive comment on “Nitrate formation from heterogeneous uptake of dinitrogen pentoxide during a severe winter haze in southern China” by Hui Yun et al. Anonymous Referee #1 Received and published: 23 September 2018 The manuscript of Yun et al., reported half month measurement of N2O5, ClNO2 and other relative parameters during heavy haze episodes in Pearl River Delta (PRD) of southern China. The N2O5 uptake coefficient and ClNO2 yield were determined from the observations. The study showed the observation evidence of the enhancement of particulate nitrate in the first several hours can be fully explained by the N2O5 het- erogeneous hydrolysis and even comparable with the nitric acid formed by OH+NO2 during daytime. Overall, the paper contributes to the knowledge of N2O5 heteroge- neous chemistry and highlight the heterogeneous reactions in the formation of partic- ulate nitrate in southern China. The following comments should be addressed before publishing on ACP. C1 Major comments: The steady state assumption to derive the N2O5 uptake coefficient needs to be verified by model simulations under the observed conditions (with input from NO, NO2, O3, VOCs). It is useful to try other method (e.g. Brown et al., 2006) to derive N2O5 uptake coefficient. Brown, S. S., Ryerson, T. B., Wollny, A. G., Brock, C. A., Peltier, R., Sullivan, A. P., Weber, R. J., Dube, W. P., Trainer, M., Meagher, J. F., Fehsenfeld, F. C., and Ravishankara, A. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride