Nitrogen Oxides (Nox), Why and How They Are Controlled
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report to the Legislature: Indoor Air Pollution in California
California Environmental Protection Agency Air Resources Board Report to the California Legislature INDOOR AIR POLLUTION IN CALIFORNIA A report submitted by: California Air Resources Board July, 2005 Pursuant to Health and Safety Code § 39930 (Assembly Bill 1173, Keeley, 2002) Arnold Schwarzenegger Governor Indoor Air Pollution in California July, 2005 ii Indoor Air Pollution in California July, 2005 ACKNOWLEDGEMENTS This report was prepared with the able and dedicated support from Jacqueline Cummins, Marisa Bolander, Jeania Delaney, Elizabeth Byers, and Heather Choi. We appreciate the valuable input received from the following groups: • Many government agency representatives who provided information and thoughtful comments on draft reports, especially Jed Waldman, Sandy McNeel, Janet Macher, Feng Tsai, and Elizabeth Katz, Department of Health Services; Richard Lam and Bob Blaisdell, Office of Environmental Health Hazard Assessment; Deborah Gold and Bob Nakamura, Cal/OSHA; Bill Pennington and Bruce Maeda, California Energy Commission; Dana Papke and Kathy Frevert, California Integrated Waste Management Board; Randy Segawa, and Madeline Brattesani, Department of Pesticide Regulation; and many others. • Bill Fisk, Lawrence Berkeley National Laboratory, for assistance in assessing the costs of indoor pollution. • Susan Lum, ARB, project website management, and Chris Jakober, for general technical assistance. • Stakeholders from the public and private sectors, who attended the public workshops and shared their experiences and suggestions -

Heat Generation Technology Landscaping Study, Scotland's
Heat Generation Technology Landscaping Study, Scotland’s Energy Efficiency Programme (SEEP) BRE August 2017 Summary This landscaping study has reviewed the status and suitability of a number of near-term heat generation technologies that are not already significantly established in the market-place. The study has been conducted to inform Scottish Government on the status and the technologies so that they can make informed decisions on the potential suitability of the technologies for inclusion within Scotland’s Energy Efficiency Programme (SEEP). Some key findings which have emerged from the research include: • High temperature, hybrid and gas driven heat pumps all have the potential to increase the uptake of low carbon heating solutions in the UK in the short to medium term. • High temperature heat pumps are particularly suited for off-gas grid retrofit projects, whereas hybrids and gas driven products are suited to on-gas grid properties. They may all be used with no or limited upgrades to existing heating systems, and each offers some advantages (but also some disadvantages) compared with standard electric heat pumps. • District heating may continue to have a significant role to play – albeit more on 3rd and 4th generation systems than the large high temperature systems typical in other parts of Europe in the 1950s and 60s – due to lower heating requirements of modern retrofitted buildings. • Longer term, the development of low carbon heating fuel markets may also present significant opportunity e.g. biogas, and possibly hydrogen. -

5Th-Generation Thermox WDG Flue Gas Combustion Analyzer
CONTROL EXCLUSIVE 5th-Generation Thermox WDG Flue Gas Combustion Analyzer “For more than 40 years, we have been a leader in combustion gas analysis,” says Mike Fuller, divison VP of sales and marketing and business development for Ametek Process Instruments, “and we believe the WDG-V is the combustion analyzer of the future.” WDG analyzers are based on a zirconium oxide cell that provides a reliable and cost-effective solution for measuring excess oxygen in flue gas as well as CO and methane levels. This information allows operators to obtain the highest fuel effi- ciency, while lowering emissions for NOx, CO and CO2. The zir- conium oxide cell responds to the difference between the concen- tration of oxygen in the flue gas versus an air reference. To assure complete combustion, the flue gas should contain several percent oxygen. The optimum excess oxygen concentration is dependent on the fuel type (natural gas, hydrocarbon liquids and coal). The WDG-V mounts directly to the process flange, and is heated to maintain all sample-wetted components above the acid dewpoint. Its air-operated aspirator draws a sample into the ana- lyzer and returns it to the process. A portion of the sample rises into the convection loop past the combustibles and oxygen cells, Ametek’s WDG-V flue gas combustion analyzer meets SIL-2 and then back into the process. standards for safety instrumented systems. This design gives a very fast response and is perfect for process heat- ers. It features a reduced-drift, hot-wire catalytic detector that is resis- analyzer with analog/HART, discrete and Modbus RS-485 bi-direc- tant to sulfur dioxide (SO ) degradation, and the instrument is suitable 2 tional communications, or supplied in a typical “sensor/controller” for process streams up to 3200 ºF (1760 ºC). -

Protein Shape Modulates Crowding Effects
Protein shape modulates crowding effects Alex J. Gusemana, Gerardo M. Perez Goncalvesa, Shannon L. Speera, Gregory B. Youngb, and Gary J. Pielaka,b,c,d,1 aDepartment of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; bDepartment of Biochemistry & Biophysics, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; cLineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; and dIntegrative Program for Biological and Genome Sciences, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 Edited by Susan Marqusee, University of California, Berkeley, CA, and approved September 12, 2018 (received for review June 18, 2018) Protein−protein interactions are usually studied in dilute buffered mildly influenced by hard-core repulsions. Berg also predicted solutions with macromolecule concentrations of <10 g/L. In cells, that more-compact dimers are more likely to be stabilized by however, the macromolecule concentration can exceed 300 g/L, hard-core repulsions. Here, we test this idea by changing the resulting in nonspecific interactions between macromolecules. shape of a dimer in a controlled fashion. These interactions can be divided into hard-core steric repulsions Scaled particle theory is based on statistical mechanics. For and “soft” chemical interactions. Here, we test a hypothesis from situations like those investigated here, the theory considers so- scaled particle theory; the influence of hard-core repulsions on a lution nonideality as arising from the presence of cosolutes (13– protein dimer depends on its shape. We tested the idea using a 15). As often applied, the solvent and cosolutes are considered side-by-side dumbbell-shaped dimer and a domain-swapped ellip- hard spheres, which leads to three consequences: Molecules only soidal dimer. -

Source Test Method ST-20 SULFUR DIOXIDE, SULFUR TRIOXIDE
Source Test Method ST-20 SULFUR DIOXIDE, SULFUR TRIOXIDE, SULFURIC ACID MIST (Adopted January 20, 1982) REF: Regulations 6-320, 6-330, 9-1-302, 9-1-304 thru 310, 10-1-301, 12-6-301 1. APPLICABILITY 1.1 This method is used to quantify emissions of s ulfur dioxide, sulfur trioxide and sulfuric acid mist. It determines compliance with Regulations 6-320 and 6-330 for SULFUR TRIOXIDE and SULFURIC ACID MIST, and 9-1-302, 9- 1-304 thru 310 and 10-1-301 and 12-6-301 for SULFUR DIOXIDE. 1.2 This method, modified with a glass fiber disc filter as the back-up SO 3 filter, has been given alternate status by the EPA to EPA Method 8. It may be used to determine compliance with oxides of sulfur regulations under Regulation 9. 2. PRINCIPLE 2.1 Sulfuric acid mist, sulfur trioxide and sulfur dioxide are collected in a single extractive sampling train. Acid mist is trapped in a quartz wool plug in the sample probe and is subsequently analyzed with an acid-base titration. Sulfur trioxide is absorbed in an 80% isopropyl alcohol (IPA)/water solution with a quartz wool back-up filter and is analyzed using analytical procedure Lab-12. Sulfur dioxide is absorbed in an aqueous hydrogen peroxide solution and is analyzed using analytical procedure Lab-12. 3. RANGE 3.1 The minimum measurable concentration using this method listed below are: 3 3.1.1 Acid mist - 0.0002 gr/ft as H2SO4 3.1.2 Sulfur trioxide - 7 ppm 3.1.3 Sulfur dioxide - 7 ppm 3.2 The maximum measurable concentrations using this method listed below are: 3.2.1 Acid mist - undetermined 3.2.2 Sulfur trioxide - 350 ppm 3.2.3 Sulfur dioxide - 2.5 % 4. -

Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11
This PDF is available from The National Academies Press at http://www.nap.edu/catalog.php?record_id=13374 Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11 ISBN Committee on Acute Exposure Guideline Levels; Committee on 978-0-309-25481-6 Toxicology; National Research Council 356 pages 6 x 9 PAPERBACK (2012) Visit the National Academies Press online and register for... Instant access to free PDF downloads of titles from the NATIONAL ACADEMY OF SCIENCES NATIONAL ACADEMY OF ENGINEERING INSTITUTE OF MEDICINE NATIONAL RESEARCH COUNCIL 10% off print titles Custom notification of new releases in your field of interest Special offers and discounts Distribution, posting, or copying of this PDF is strictly prohibited without written permission of the National Academies Press. Unless otherwise indicated, all materials in this PDF are copyrighted by the National Academy of Sciences. Request reprint permission for this book Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11 Committee on Acute Exposure Guideline Levels Committee on Toxicology Board on Environmental Studies and Toxicology Division on Earth and Life Studies Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 11 THE NATIONAL ACADEMIES PRESS 500 FIFTH STREET, NW WASHINGTON, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Insti- tute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance. -

Dimerization of Carboxylic Acids: an Equation of State Approach
Downloaded from orbit.dtu.dk on: Oct 05, 2021 Dimerization of Carboxylic Acids: An Equation of State Approach Tsivintzelis, Ioannis; Kontogeorgis, Georgios; Panayiotou, Costas Published in: Journal of Physical Chemistry Part B: Condensed Matter, Materials, Surfaces, Interfaces & Biophysical Link to article, DOI: 10.1021/acs.jpcb.6b10652 Publication date: 2017 Document Version Peer reviewed version Link back to DTU Orbit Citation (APA): Tsivintzelis, I., Kontogeorgis, G., & Panayiotou, C. (2017). Dimerization of Carboxylic Acids: An Equation of State Approach. Journal of Physical Chemistry Part B: Condensed Matter, Materials, Surfaces, Interfaces & Biophysical, 121(9), 2153-2163. https://doi.org/10.1021/acs.jpcb.6b10652 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. On the Dimerization of Carboxylic Acids: An Equation of State Approach Ioannis Tsivintzelis*,1, Georgios M. Kontogeorgis2 and Costas Panayiotou1 1Department of Chemical Engineering, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece. 2Center for Energy Resources Engineering (CERE), Department of Chemical and Biochemical Engineering, Technical University of Denmark, DK-2800 Kgs. -

AP Chemistry Free Response
AP Chemistry Exam Reactions: Questions and Answers With the new format of the exam in 2007 and the availability of both questions and answers on the web at AP Central (http://apcentral.collegeboard.com:80/apc/public/courses/4606.html), I have determined not to update this page any longer. Please create an account as a teacher at AP Central and navigate to the full exams and scoring rubrics which are available back to 2003 Beginning in 2007, question 4 is no longer 5 out of 8 responses but rather three required responses. Also, in addition to writing the reactants and products, the equation must be balanced and there is a question about the chemical reaction. 2007 (a) A solution of sodium hydroxide is added to a solution of lead(II) nitrate. If 1.0 L volumes of 1.0 M solutions of sodium hydroxide and lead(II) nitrate are mixed together, now many moles of product(s) will be produced? Assume the reaction goes to completion. (b) Excess nitric acid is added to solid calcium carbonate. Briefly explain why statues made of marble (calcium carbonate) displayed outdoors in urban areas are deteriorating. (c) A solution containing silver(I) ion (an oxidixing agent) is mixed with a; solution containing iron(II) ion (a reducing agent). If the contents of the reaction mixture described above are filtered, what substance(s), if any, would remain on the filter paper? - 2+ → (a) (i) Balanced equation: 2OH + Pb Pb(OH)2 (s) (ii) The moles of each reactant are obtained by multiplying the volume times the molarity. -

Nitrogen Oxide - Wikipedia, the Free Encyclopedia Page 1 of 3
Nitrogen oxide - Wikipedia, the free encyclopedia Page 1 of 3 Nitrogen oxide From Wikipedia, the free encyclopedia Nitrogen oxide can refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds: Contents ■ Nitric oxide (NO), nitrogen(II) oxide ■ Nitrogen dioxide (NO2), nitrogen(IV) oxide ■ 1 NOx ■ Nitrous oxide (N2O), nitrogen(I) oxide ■ 2 Derivatives ■ Nitrate radical (NO3), nitrogen(VI) oxide ■ 3 See also ■ Dinitrogen trioxide (N2O3), nitrogen(II,IV) oxide ■ 4 References ■ Dinitrogen tetroxide (N2O4), nitrogen(IV) oxide ■ Dinitrogen pentoxide (N2O5), nitrogen(V) oxide In atmospheric chemistry and air pollution and related fields, nitrogen oxides refers specifically to NOx [1][2] (NO and NO2). Only the first three of these compounds can be isolated at room temperature. N2O3, N2O4, and N2O5 all decompose rapidly at room temperature. Nitrate radical is very reactive. N2O is stable and rather unreactive at room temperature, while NO and NO2 are quite reactive but nevertheless quite stable when isolated. Dinitrogen trioxide, Nitric oxide, NO Nitrogen dioxide, NO2 Nitrous oxide, N2O N2O3 Dinitrogen tetroxide, Dinitrogen pentoxide, N2O4 N2O5 http://en.wikipedia.org/wiki/Nitrogen_oxide 11/2/2010 Nitrogen oxide - Wikipedia, the free encyclopedia Page 2 of 3 NOx Main article: NOx NOx (often written NOx) refers to NO and NO2. They are produced during combustion, especially at high temperature. These two chemicals are important trace species in Earth's atmosphere. In the troposphere, during daylight, NO reacts with partly oxidized organic species (or the peroxy radical) to form NO2, which is then photolyzed by sunlight to reform NO: NO + CH3O2 → NO2 + CH3O NO2 + sunlight → NO + O The oxygen atom formed in the second reaction then goes on to form ozone; this series of reactions is the main source of tropospheric ozone. -

Ocean Storage
277 6 Ocean storage Coordinating Lead Authors Ken Caldeira (United States), Makoto Akai (Japan) Lead Authors Peter Brewer (United States), Baixin Chen (China), Peter Haugan (Norway), Toru Iwama (Japan), Paul Johnston (United Kingdom), Haroon Kheshgi (United States), Qingquan Li (China), Takashi Ohsumi (Japan), Hans Pörtner (Germany), Chris Sabine (United States), Yoshihisa Shirayama (Japan), Jolyon Thomson (United Kingdom) Contributing Authors Jim Barry (United States), Lara Hansen (United States) Review Editors Brad De Young (Canada), Fortunat Joos (Switzerland) 278 IPCC Special Report on Carbon dioxide Capture and Storage Contents EXECUTIVE SUMMARY 279 6.7 Environmental impacts, risks, and risk management 298 6.1 Introduction and background 279 6.7.1 Introduction to biological impacts and risk 298 6.1.1 Intentional storage of CO2 in the ocean 279 6.7.2 Physiological effects of CO2 301 6.1.2 Relevant background in physical and chemical 6.7.3 From physiological mechanisms to ecosystems 305 oceanography 281 6.7.4 Biological consequences for water column release scenarios 306 6.2 Approaches to release CO2 into the ocean 282 6.7.5 Biological consequences associated with CO2 6.2.1 Approaches to releasing CO2 that has been captured, lakes 307 compressed, and transported into the ocean 282 6.7.6 Contaminants in CO2 streams 307 6.2.2 CO2 storage by dissolution of carbonate minerals 290 6.7.7 Risk management 307 6.2.3 Other ocean storage approaches 291 6.7.8 Social aspects; public and stakeholder perception 307 6.3 Capacity and fractions retained -
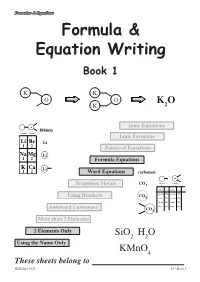
Formula & Equation Writing
Formulae & Equations Formula & Equation Writing Book 1 K K O O K O K 2 Ionic Equations lithium column column 1 2 Ionic Formulae Li Be Li 1 2 Balanced Equations Na Mg Li 1 2 Formula Equations K Ca Li 1 2 Word Equations carbonate valency valency Transition Metals CO3 1 2 name formula name formula ammonium NH4 carbonate CO3 Using Brackets CO 3 cyanide CN chromate CrO4 hydroxide OH sulphate SO4 nitrate NO sulphite SO Awkward Customers 3 3 CO3 More than 2 Elements 2 Elements Only SiO2 H2O Using the Name Only KMnO4 These sheets belong to KHS Sept 2013 page 1 S3 - Book 1 Formulae & Equations What is a Formula ? The formula of a compound tells you two things about the compound :- SiO H O i) which elements are in the compound 2 2 using symbols, and KMnO4 ii) how many atoms of each element are in the compound using numbers. Test Yourself What would be the formula for each of the following? Br H Cl K H C S Al Br O H Cl K H Br Using the Name Only Some compounds have extra information in their carbon monoxide names that allow people to work out and write CO the correct formula. carbon dioxide CO2 The names of the elements appear as usual but dinitrogen tetroxide N O this time the number of each type of atom is 2 4 included using mono- = 1 di- = 12 tri- = 3 tetra- = 4 penta- = 5 hexa- =46 Test Yourself 1 What would be the formula for each of the following? 1. -

The Chemical Behaviors of Nitrogen Dioxide Absorption in Sulfite Solution
Article The Chemical Behaviors of Nitrogen Dioxide Absorption in Sulfite Solution Ye Sun, Xiaowei Hong, Tianle Zhu *, Xiaoyan Guo and Deyuan Xie School of Space and Environment, Beihang University, Beijing 100191, China; [email protected] (Y.S.); [email protected] (X.H.); [email protected] (X.G.); [email protected] (D.X.) * Correspondence: [email protected]; Tel.: +86-10-8231-4215 Academic Editor: Agustín Bueno López Received: 14 March 2017; Accepted: 6 April 2017; Published: 12 April 2017 Abstract: The simultaneous removal of nitrogen oxides (NOx) and sulfur dioxide (SO2) by absorption is considered to be one of the most promising technologies for flue gas treatment, and sulfite is the main component of the absorption solution. To understand the chemical behaviors of the NO2 absorption in sulfite solution, the absorption time dependences of concentrations of nitrogen and sulfur compositions in both gas phase and liquid phases were investigated by flue gas analyzer, Ion chromatography (IC), Gas chromatography (GC), and Fourier transform infrared spectrometry (FTIR) methods using a bubbling reactor. The mass equilibrium of the N and S compositions were also studied. The results indicate that sulfite concentration plays a vital role in NO2 absorption. The main absorption products are NO3− and NO2−, and NO2− can be converted into NO3− in the presence of oxygen. Besides, about 4% to 9% by-products of S compositions are formed, and 4% to 11% by-products of N compositions such as NO, N2, N2O5, N2O, and HNO3 in the gas phase were detected in the emissions from the bubbling reactor. On the basis of N and S compositions, a possible pathway of NO2 absorption in sulfite solution was proposed.