Polymorphism, Halogen Bonding, and Chalcogen Bonding in the Diiodine Adducts of 1,3- and 1,4-Dithiane
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protein Shape Modulates Crowding Effects
Protein shape modulates crowding effects Alex J. Gusemana, Gerardo M. Perez Goncalvesa, Shannon L. Speera, Gregory B. Youngb, and Gary J. Pielaka,b,c,d,1 aDepartment of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; bDepartment of Biochemistry & Biophysics, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; cLineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; and dIntegrative Program for Biological and Genome Sciences, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 Edited by Susan Marqusee, University of California, Berkeley, CA, and approved September 12, 2018 (received for review June 18, 2018) Protein−protein interactions are usually studied in dilute buffered mildly influenced by hard-core repulsions. Berg also predicted solutions with macromolecule concentrations of <10 g/L. In cells, that more-compact dimers are more likely to be stabilized by however, the macromolecule concentration can exceed 300 g/L, hard-core repulsions. Here, we test this idea by changing the resulting in nonspecific interactions between macromolecules. shape of a dimer in a controlled fashion. These interactions can be divided into hard-core steric repulsions Scaled particle theory is based on statistical mechanics. For and “soft” chemical interactions. Here, we test a hypothesis from situations like those investigated here, the theory considers so- scaled particle theory; the influence of hard-core repulsions on a lution nonideality as arising from the presence of cosolutes (13– protein dimer depends on its shape. We tested the idea using a 15). As often applied, the solvent and cosolutes are considered side-by-side dumbbell-shaped dimer and a domain-swapped ellip- hard spheres, which leads to three consequences: Molecules only soidal dimer. -

An Alternate Graphical Representation of Periodic Table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt
An Alternate Graphical Representation of Periodic table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt. Ltd, Hyderabad, India. [email protected] Abstract Periodic table of chemical elements symbolizes an elegant graphical representation of symmetry at atomic level and provides an overview on arrangement of electrons. It started merely as tabular representation of chemical elements, later got strengthened with quantum mechanical description of atomic structure and recent studies have revealed that periodic table can be formulated using SO(4,2) SU(2) group. IUPAC, the governing body in Chemistry, doesn‟t approve any periodic table as a standard periodic table. The only specific recommendation provided by IUPAC is that the periodic table should follow the 1 to 18 group numbering. In this technical paper, we describe a new graphical representation of periodic table, referred as „Circular form of Periodic table‟. The advantages of circular form of periodic table over other representations are discussed along with a brief discussion on history of periodic tables. 1. Introduction The profoundness of inherent symmetry in nature can be seen at different depths of atomic scales. Periodic table symbolizes one such elegant symmetry existing within the atomic structure of chemical elements. This so called „symmetry‟ within the atomic structures has been widely studied from different prospects and over the last hundreds years more than 700 different graphical representations of Periodic tables have emerged [1]. Each graphical representation of chemical elements attempted to portray certain symmetries in form of columns, rows, spirals, dimensions etc. Out of all the graphical representations, the rectangular form of periodic table (also referred as Long form of periodic table or Modern periodic table) has gained wide acceptance. -

25 WORDS CHLORINE Chlorine, Cl, Is a Very Poisonous Green Gas That's
25 WORDS CHLORINE Chlorine, Cl, is a very poisonous green gas that's extremely reactive. It's used for sanitizing, purifying, and was used as a weapon during World War I by the Germans. But in chemistry, it is an oxidizer. Chlorine, Cl, is a green gaseous element with an atomic number of 17. This halogen is a powerful oxidant and used to produce many things, such as cleaning products. Chlorine; it's chemical symbol is Cl. Chloride is abundant in nature and necessary for life but a large amount can cause choking and and poisoning. It's mainly used for water purification but has other uses. Chlorine is a halogen and to test if it has a halogen, we use the Beilstein Copper Wire Test. It is also used to produce safe drinking water. Chlorine, atomic number seventeen, is a halogen that is found in table salt, NaCl, making it essential to life. However, pure chlorine, Cl2, is a poisonous gas, detectable at even 1 ppm. Chlorine, (Symbol Cl), belongs to the halogen family of elements, found in group 17 on the periodic table. Chlorine has an atomic number of 17 and atomic weight of 35.453. Chlorine is the 17th element on the periodic table, and is in the "Halogens" group, which has a tendency to gain one electron to form anions. Its anion can be found commonly in table salt Chlorine (symbolized Cl) is the chemical element with atomic number 17. Clorine is a powerful oxidant and is used in bleaching and disinfectants. It is a pale yellow-green gas that has a specific strong smell. -

Driving Halogen Lamps Application Note
Application Note 1604 Driving Halogen Lamps Abstract: This application note looks at the suitability of the Ultimod wide trim powerMods for applications driving Halogen lamps. An incandescent lamp generates light by heating is that the powerMod will go into a protective a tungsten wire or filament until it glows (at current limit. The characteristics of this current around 2,500 ºC) by passing an electric current limit are shown in Figure 1 below. through it. A halogen lamp is basically a modified version of an incandescent lamp. The difference is that the bulb of a halogen lamp has a small amount of a halogen gas added. The presence of this halogen in the bulb produces a chemical reaction (known as the halogen cycle) that redeposists tungsten evaporated by heating back onto the filament. In a standard incandescent the constant evaporation leads to the eventual failure of the lamp as the filament progressively thins and breaks or “burns out”. Since in a halogen lamp the tungsten is redeposited back on the filament Figure 1 Current Limit Characteristics its lifetime is extended, and it also be heated to a higher temperature (in the region of 3,000 ºC), which increases its efficiency. You can see from Figure 1 that when we increase the load (by reducing the loads The high operational temperature of the filament resistance) and the current increases above the results in a challenge for a constant voltage set current limit of the modules we enter a mode power supplies like the Ultimod due to the of operation known as straight line current different resistance of the tungsten filament limiting where the current is held constant and at room temperature and its resistance at 3,000 the voltage is reduced. -

Dimerization of Carboxylic Acids: an Equation of State Approach
Downloaded from orbit.dtu.dk on: Oct 05, 2021 Dimerization of Carboxylic Acids: An Equation of State Approach Tsivintzelis, Ioannis; Kontogeorgis, Georgios; Panayiotou, Costas Published in: Journal of Physical Chemistry Part B: Condensed Matter, Materials, Surfaces, Interfaces & Biophysical Link to article, DOI: 10.1021/acs.jpcb.6b10652 Publication date: 2017 Document Version Peer reviewed version Link back to DTU Orbit Citation (APA): Tsivintzelis, I., Kontogeorgis, G., & Panayiotou, C. (2017). Dimerization of Carboxylic Acids: An Equation of State Approach. Journal of Physical Chemistry Part B: Condensed Matter, Materials, Surfaces, Interfaces & Biophysical, 121(9), 2153-2163. https://doi.org/10.1021/acs.jpcb.6b10652 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. On the Dimerization of Carboxylic Acids: An Equation of State Approach Ioannis Tsivintzelis*,1, Georgios M. Kontogeorgis2 and Costas Panayiotou1 1Department of Chemical Engineering, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece. 2Center for Energy Resources Engineering (CERE), Department of Chemical and Biochemical Engineering, Technical University of Denmark, DK-2800 Kgs. -

Spin Delocalization, Polarization, and London Dispersion Forces Govern the Formation of Diradical Pimers
Chemistry Publications Chemistry 2-22-2020 Spin Delocalization, Polarization, and London Dispersion Forces Govern the Formation of Diradical Pimers Joshua P. Peterson Iowa State University, [email protected] Arkady Ellern Iowa State University, [email protected] Arthur H. Winter Iowa State University, [email protected] Follow this and additional works at: https://lib.dr.iastate.edu/chem_pubs Part of the Chemistry Commons The complete bibliographic information for this item can be found at https://lib.dr.iastate.edu/ chem_pubs/1215. For information on how to cite this item, please visit http://lib.dr.iastate.edu/ howtocite.html. This Article is brought to you for free and open access by the Chemistry at Iowa State University Digital Repository. It has been accepted for inclusion in Chemistry Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Spin Delocalization, Polarization, and London Dispersion Forces Govern the Formation of Diradical Pimers Abstract Some free radicals are stable enough to be isolated, but most are either unstable transient species or exist as metastable species in equilibrium with a dimeric form, usually a spin-paired sigma dimer or a pi dimer (pimer). To gain insight into the different modes of dimerization, we synthesized and evaluated a library of 15 aryl dicyanomethyl radicals in order to probe what structural and molecular parameters lead to σ- versus π-dimerization. We evaluated the divergent dimerization behavior by measuring the strength of each radical association by variable-temperature electron paramagnetic resonance spectroscopy, determining the mode of dimerization (σ- or π-dimer) by UV–vis spectroscopy and X-ray crystallography, and performing computational analysis. -

Of the Periodic Table
of the Periodic Table teacher notes Give your students a visual introduction to the families of the periodic table! This product includes eight mini- posters, one for each of the element families on the main group of the periodic table: Alkali Metals, Alkaline Earth Metals, Boron/Aluminum Group (Icosagens), Carbon Group (Crystallogens), Nitrogen Group (Pnictogens), Oxygen Group (Chalcogens), Halogens, and Noble Gases. The mini-posters give overview information about the family as well as a visual of where on the periodic table the family is located and a diagram of an atom of that family highlighting the number of valence electrons. Also included is the student packet, which is broken into the eight families and asks for specific information that students will find on the mini-posters. The students are also directed to color each family with a specific color on the blank graphic organizer at the end of their packet and they go to the fantastic interactive table at www.periodictable.com to learn even more about the elements in each family. Furthermore, there is a section for students to conduct their own research on the element of hydrogen, which does not belong to a family. When I use this activity, I print two of each mini-poster in color (pages 8 through 15 of this file), laminate them, and lay them on a big table. I have students work in partners to read about each family, one at a time, and complete that section of the student packet (pages 16 through 21 of this file). When they finish, they bring the mini-poster back to the table for another group to use. -

UNITED STATES PATENT OFFICE 2,680,133 ODNE-CONTAINING AMNO-BENZOY, DERVATIVES of AMNO ACDS Vernon H
Patented June 1, 1954 2,680,133 UNITED STATES PATENT OFFICE 2,680,133 ODNE-CONTAINING AMNO-BENZOY, DERVATIVES OF AMNO ACDS Vernon H. Wallingford, Ferguson, Mo., assignor to Mallinckrodt Chemical Works, St. Louis, Mo, a corporation of Missouri No Drawing. Application August 24, 1951, Serial No. 243,577 8 Claims. (C. 260-518) 2 This invention relates to iodine-containing proposed as X-ray contrast agents; but only a few amino-benzoyl derivatives of amino acids and of these are now recognized as being of any prac more particularly to 3-amino-2,4,6-triiodo deriva tical value. The problem remains of providing tives of benzoyl amino acid compounds and to a high degree of contrast for X-ray diagnosis with methods for their preparation. greater safety and comfort to the patient. This This application is a continuation-in-part of can be achieved, for example, by providing con my copending application Serial No. 94,253, filed trast agents that are (1) less toxic, so that larger May 19, 1949, now abandoned. amounts can be given to the patient; (2) more Briefly the invention comprises methods of soluble, so that greater concentrations of the con making certain compounds of a group having the O trast agent are possible; or (3) more opaque to formula: X-rays because of a greater proportion of iodine CO-N-R-COOH but without a corresponding increase in toxicity. ly Iodinated derivatives of benzoic acid are among I- I those compounds that have been proposed as 15 X-ray contrast agents. Although they appear to NE be promising X-ray contrast agents because of the large amount of iodine that they contain, the toxicity or the in Solubility of the known deriva tives of these compounds have generally been where R is selected from the group consisting of 20 found to be too great for this purpose. -

Nuclear Substitution Reactions of Dibenzo-P-Dioxin Joseph Jacob Dietrich Iowa State College
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1957 Nuclear substitution reactions of dibenzo-p-dioxin Joseph Jacob Dietrich Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Dietrich, Joseph Jacob, "Nuclear substitution reactions of dibenzo-p-dioxin " (1957). Retrospective Theses and Dissertations. 1336. https://lib.dr.iastate.edu/rtd/1336 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. NUCLEAR SUBSTITUTION REACTIONS OF DI3ENZC-£-BIOXIN by- Joseph Jacob Dietrich A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of 'DOCTOR OF PHILOSOPHY Major Subject : Organic Chemistry Approved: Signature was redacted for privacy. in Charge of I-'*ajor *.7ork Signature was redacted for privacy. Head of Ma nartment Signature was redacted for privacy. Dean of Graduate College Io?;a State College 1957 11 TABLE OF CONTENTS Page INTRODUCTION 1 -ISTJRICAL 5 Dibenzo-n-dioxin 5 Alkyl Derivatives Ô Carbonyl Derivatives 8 Carbozyl Derivatives 9 Dithiocarboxylate Derivatives 10 Halogen Derivatives 10 Hydroxy Derivatives 12 Methoxy Derivatives -

IODINE Its Properties and Technical Applications
IODINE Its Properties and Technical Applications CHILEAN IODINE EDUCATIONAL BUREAU, INC. 120 Broadway, New York 5, New York IODINE Its Properties and Technical Applications ¡¡iiHiüíiüüiütitittüHiiUitítHiiiittiíU CHILEAN IODINE EDUCATIONAL BUREAU, INC. 120 Broadway, New York 5, New York 1951 Copyright, 1951, by Chilean Iodine Educational Bureau, Inc. Printed in U.S.A. Contents Page Foreword v I—Chemistry of Iodine and Its Compounds 1 A Short History of Iodine 1 The Occurrence and Production of Iodine ....... 3 The Properties of Iodine 4 Solid Iodine 4 Liquid Iodine 5 Iodine Vapor and Gas 6 Chemical Properties 6 Inorganic Compounds of Iodine 8 Compounds of Electropositive Iodine 8 Compounds with Other Halogens 8 The Polyhalides 9 Hydrogen Iodide 1,0 Inorganic Iodides 10 Physical Properties 10 Chemical Properties 12 Complex Iodides .13 The Oxides of Iodine . 14 Iodic Acid and the Iodates 15 Periodic Acid and the Periodates 15 Reactions of Iodine and Its Inorganic Compounds With Organic Compounds 17 Iodine . 17 Iodine Halides 18 Hydrogen Iodide 19 Inorganic Iodides 19 Periodic and Iodic Acids 21 The Organic Iodo Compounds 22 Organic Compounds of Polyvalent Iodine 25 The lodoso Compounds 25 The Iodoxy Compounds 26 The Iodyl Compounds 26 The Iodonium Salts 27 Heterocyclic Iodine Compounds 30 Bibliography 31 II—Applications of Iodine and Its Compounds 35 Iodine in Organic Chemistry 35 Iodine and Its Compounds at Catalysts 35 Exchange Catalysis 35 Halogenation 38 Isomerization 38 Dehydration 39 III Page Acylation 41 Carbón Monoxide (and Nitric Oxide) Additions ... 42 Reactions with Oxygen 42 Homogeneous Pyrolysis 43 Iodine as an Inhibitor 44 Other Applications 44 Iodine and Its Compounds as Process Reagents ... -
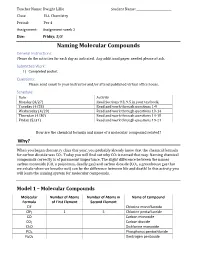
Naming Molecular Compounds General Instructions: Please Do the Activities for Each Day As Indicated
Teacher Name: Dwight Lillie Student Name: ________________________ Class: ELL Chemistry Period: Per 4 Assignment: Assignment week 2 Due: Friday, 5/8 Naming Molecular Compounds General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Submitted Work: 1) Completed packet. Questions: Please send email to your instructor and/or attend published virtual office hours. Schedule: Date Activity Monday (4/27) Read Sections 9.3, 9.5 in your textbook. Tuesday (4/28) Read and work through questions 1-9 Wednesday (4/29) Read and work through questions 10-14 Thursday (4/30) Read and work through questions 14-18 Friday (5/31) Read and work through questions 19-21 How are the chemical formula and name of a molecular compound related? Why? When you began chemistry class this year, you probably already knew that the chemical formula for carbon dioxide was CO2. Today you will find out why CO2 is named that way. Naming chemical compounds correctly is of paramount importance. The slight difference between the names carbon monoxide (CO, a poisonous, deadly gas) and carbon dioxide (CO2, a greenhouse gas that we exhale when we breathe out) can be the difference between life and death! In this activity you will learn the naming system for molecular compounds. Model 1 – Molecular Compounds Molecular Number of Atoms Number of Atoms in Name of Compound Formula of First Element Second Element ClF Chlorine monofluoride ClF5 1 5 Chlorine pentafluoride CO Carbon monoxide CO2 Carbon dioxide Cl2O Dichlorine monoxide PCl5 Phosphorus pentachloride N2O5 Dinitrogen pentoxide 1. Fill in the table to indicate the number of atoms of each type in the molecular formula. -

Step-By-Step Guide to Better Laboratory Management Practices
Step-by-Step Guide to Better Laboratory Management Practices Prepared by The Washington State Department of Ecology Hazardous Waste and Toxics Reduction Program Publication No. 97- 431 Revised January 2003 Printed on recycled paper For additional copies of this document, contact: Department of Ecology Publications Distribution Center PO Box 47600 Olympia, WA 98504-7600 (360) 407-7472 or 1 (800) 633-7585 or contact your regional office: Department of Ecology’s Regional Offices (425) 649-7000 (509) 575-2490 (509) 329-3400 (360) 407-6300 The Department of Ecology is an equal opportunity agency and does not discriminate on the basis of race, creed, color, disability, age, religion, national origin, sex, marital status, disabled veteran’s status, Vietnam Era veteran’s status or sexual orientation. If you have special accommodation needs, or require this document in an alternate format, contact the Hazardous Waste and Toxics Reduction Program at (360)407-6700 (voice) or 711 or (800) 833-6388 (TTY). Table of Contents Introduction ....................................................................................................................................iii Section 1 Laboratory Hazardous Waste Management ...........................................................1 Designating Dangerous Waste................................................................................................1 Counting Wastes .......................................................................................................................8 Treatment by Generator...........................................................................................................12