The Multiphase Chemical Kinetics of Dinitrogen Pentoxide with Aerosol Particles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nitrogen Oxide - Wikipedia, the Free Encyclopedia Page 1 of 3
Nitrogen oxide - Wikipedia, the free encyclopedia Page 1 of 3 Nitrogen oxide From Wikipedia, the free encyclopedia Nitrogen oxide can refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds: Contents ■ Nitric oxide (NO), nitrogen(II) oxide ■ Nitrogen dioxide (NO2), nitrogen(IV) oxide ■ 1 NOx ■ Nitrous oxide (N2O), nitrogen(I) oxide ■ 2 Derivatives ■ Nitrate radical (NO3), nitrogen(VI) oxide ■ 3 See also ■ Dinitrogen trioxide (N2O3), nitrogen(II,IV) oxide ■ 4 References ■ Dinitrogen tetroxide (N2O4), nitrogen(IV) oxide ■ Dinitrogen pentoxide (N2O5), nitrogen(V) oxide In atmospheric chemistry and air pollution and related fields, nitrogen oxides refers specifically to NOx [1][2] (NO and NO2). Only the first three of these compounds can be isolated at room temperature. N2O3, N2O4, and N2O5 all decompose rapidly at room temperature. Nitrate radical is very reactive. N2O is stable and rather unreactive at room temperature, while NO and NO2 are quite reactive but nevertheless quite stable when isolated. Dinitrogen trioxide, Nitric oxide, NO Nitrogen dioxide, NO2 Nitrous oxide, N2O N2O3 Dinitrogen tetroxide, Dinitrogen pentoxide, N2O4 N2O5 http://en.wikipedia.org/wiki/Nitrogen_oxide 11/2/2010 Nitrogen oxide - Wikipedia, the free encyclopedia Page 2 of 3 NOx Main article: NOx NOx (often written NOx) refers to NO and NO2. They are produced during combustion, especially at high temperature. These two chemicals are important trace species in Earth's atmosphere. In the troposphere, during daylight, NO reacts with partly oxidized organic species (or the peroxy radical) to form NO2, which is then photolyzed by sunlight to reform NO: NO + CH3O2 → NO2 + CH3O NO2 + sunlight → NO + O The oxygen atom formed in the second reaction then goes on to form ozone; this series of reactions is the main source of tropospheric ozone. -

UNIVERSITY of CALIFORNIA, SAN DIEGO Triple Oxygen Isotope
UNIVERSITY OF CALIFORNIA, SAN DIEGO Triple Oxygen Isotope Measurement of Nitrate to Analyze Impact of Aircraft Emissions A Thesis submitted in partial satisfaction of the requirements for the degree Master of Science in Chemistry by Sharleen Chan Committee in charge: Professor Mark Thiemens, Chair Professor Timothy Bertram Professor William Trogler 2013 Copyright Sharleen Chan, 2013 All rights reserved. The Thesis of Sharleen Chan is approved and it is acceptable in quality and form for publication on microfilm and electronically: ___________________________________________ ___________________________________________ ___________________________________________ Chair University of California, San Diego 2013 iii DEDICATION To my family whose love, inspiration, and support made the completion of my degree possible. iv TABLE OF CONTENTS Signature Page………………………………………………..…………………………. iii Dedication……………………………………………………..………………..……….. iv Table of Contents…………………………………………………….………….….……. v List of Figures……………………………..………………………………………....…. vii List of Tables………………………………………………………………...………….. ix Acknowledgements………………………………………………..……….…………..… x Abstract………………………………………………………………...…......……....…. xi Chapter 1 Nitrate and the Nitrogen Cycle………………………………….………......… 1 1.1 The Nitrogen Cycle………………………………………………..........……...… 1 1.2 Atmospheric nitrogen species in the Troposphere………………………......…… 1 1.2.1 Leighton relationship…………………………………...…...…… 4 1.3 Consequences of anthropogenic nitrogen emissions……………….….………… 4 1.4 References……………………………………………………...………………… -

Analysis of Tropospheric Trace Gas Amounts from Satellite and Ship-Based DOAS-Type Measurements
Analysis of tropospheric trace gas amounts from satellite and ship-based DOAS-type measurements: NO2 from biomass burning and other sources Mag.rer.nat. Ing. Stefan Friedrich Schreier Dissertation zur Erlangung des Grades Doktor der Naturwissenschaften (Dr.rer.nat.) Institut für Umweltphysik Fachbereich Physik und Elektrotechnik Universität Bremen Oktober 2014 1. Gutachter: Prof. Dr. John P. Burrows 2. Gutachter: Prof. Dr. Justus Notholt Betreuer: Dr. Andreas Richter Dissertation eingereicht am: 13. Oktober 2014 Datum des Kolloquiums: 11. Dezember 2014 ii Du kannst dein Leben nicht verlängern und du kannst es auch nicht verbreitern. Aber du kannst es vertiefen. Gorch Fock, 1880-1916 iii iv Abstract Nitrogen oxides (NOx) play key roles in atmospheric chemistry, air pollution, and climate. While the largest fraction of these reactive gases is released by anthropogenic emission sources, a significant amount can be attributed to vegetation fires. Tropospheric nitrogen dioxide (NO2) amounts can be retrieved from ground-based, ship-based, aircraft-based, and satellite-based remote sensing measurements. The focus of this thesis is to analyze such NO2 measurements for the characterization of NOx from open biomass burning and other sources. In the first part of this thesis, satellite measurements of tropospheric NO2 from GOME-2 and OMI as well as fire radiative power (FRP) from the MODIS instruments are used to derive seasonally averaged fire emission rates (FERs) of NOx for different types of vegetation using a simple statistical approach. Monthly means of tropospheric NO2 vertical columns (TVC NO2) are analyzed for their temporal correlation with the monthly means of FRP for a multi-year period. -

COVALENT COMPOUNDS Unit 2 COVALENT BOND
COVALENT COMPOUNDS Unit 2 COVALENT BOND Forms between two non-metals! Electrons are shared! Co= share Valent = electrons Since electrons are shared, no need to balance charges Covalent bonds form molecules. Molecule - group of neutral atoms held together with a covalent bond Diatomic molecules – molecule consisting of 2 atoms MOLECULES H2O2Br2F2I2N2Cl2 Compound composed of molecules is a molecular compound. MOLECULAR COMPOUNDS Molecular Compounds Properties Gases or liquids Low melting/boiling points Usually made from 2 nonmetals bonded covalently Do not conduct electricity (solid or dissolved) Molecular chemical formula shows how many atoms of each element a molecule contains. H2O – Molecular formula of water 2 Hydrogen atoms, 1 Oxygen atom CO2 – Molecular formula for Carbon Dioxide MOLECULAR 1 Carbon atom, 2 Oxygen atoms FORMULAS H:H - Lewis Dot H-H – Structural Formula H2 – Molecular chemical formula LEWIS STRUCTURES COVALENT BONDING To draw a Lewis Structure, one must know the types of atoms in the molecule, the number of atoms in the molecule and the number of valence electrons for each atom in the molecule. DOUBLE & TRIPLE COVALENT BONDS Sharing a pair of electrons creates a single covalent bond Sharing more than one pair of electrons creates double and triple covalent bonds. 2 shared pairs – Double covalent bond 3 shared pairs – Triple covalent bond Carbon, Nitrogen, Oxygen 1. First element is named first, using the full name of the element 2. The second element is named by dropping ending and adding RULES FOR “–ide” NAMING 3. Prefixes are used to denote # COVALENT of each atom present COMPOUNDS 4. Prefix mono- is never used on the first element 5. -
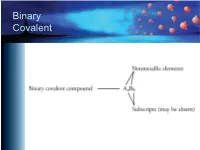
Binary Covalent Common Names
Binary Covalent Common Names – H2O, water – NH3, ammonia – CH4, methane – C2H6, ethane – C3H8, propane – C4H10, butane – C5H12, pentane – C6H14, hexane Naming Binary Covalent CompounDs • If the subscript for the first element is greater than one, inDicate the subscript with a prefix. – We Do not write mono- on the first name. – Leave the "a" off the enD of the prefixes that enD in "a" anD the “o” off of mono- if they are placeD in front of an element that begins with a vowel (oxygen or ioDine). Prefixes mon(o) hex(a) di hept(a) tri oct(a) tetr(a) non(a) pent(a) dec(a) Nitrogen OxiDe Names • N2O3 – name starts with di • N2O5 – name starts with di • NO2 – no initial prefix • NO – no initial prefix Naming Binary Covalent CompounDs • Follow the prefix with the name of the first element in the formula. – N2O3 – dinitrogen – N2O3 – dinitrogen – NO2 – nitrogen – NO – nitrogen Naming Binary Covalent CompounDs • Write a prefix to inDicate the subscript for the seconD element. (Remember to leave the “o” off of mono- anD the “a” off of the prefixes that enD in “a” when they are placeD in front of a name that begins with a vowel.) – N2O3 – dinitrogen tri – N2O5 – dinitrogen pent – NO2 – nitrogen di – NO – nitrogen mon Naming Binary Covalent CompounDs • Write the root of the name of the seconD symbol in the formula. (See the next sliDe.) – N2O3 – dinitrogen triox – N2O5 – dinitrogen pentox – NO2 – nitrogen diox – NO – nitrogen monox Roots of Nonmetals H hydr- F fluor- C carb- Cl chlor- N nitr- Br brom- P phosph- I iod- O ox- S sulf- Se selen- Naming Binary Covalent CompounDs • AdD -ide to the enD of the name. -

Nitrate Formation from Heterogeneous Uptake of Dinitrogen Pentoxide During a Severe Winter Haze in Southern China” by Hui Yun Et Al
Atmos. Chem. Phys. Discuss., https://doi.org/10.5194/acp-2018-698-RC1, 2018 © Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Interactive comment on “Nitrate formation from heterogeneous uptake of dinitrogen pentoxide during a severe winter haze in southern China” by Hui Yun et al. Anonymous Referee #1 Received and published: 23 September 2018 The manuscript of Yun et al., reported half month measurement of N2O5, ClNO2 and other relative parameters during heavy haze episodes in Pearl River Delta (PRD) of southern China. The N2O5 uptake coefficient and ClNO2 yield were determined from the observations. The study showed the observation evidence of the enhancement of particulate nitrate in the first several hours can be fully explained by the N2O5 het- erogeneous hydrolysis and even comparable with the nitric acid formed by OH+NO2 during daytime. Overall, the paper contributes to the knowledge of N2O5 heteroge- neous chemistry and highlight the heterogeneous reactions in the formation of partic- ulate nitrate in southern China. The following comments should be addressed before publishing on ACP. C1 Major comments: The steady state assumption to derive the N2O5 uptake coefficient needs to be verified by model simulations under the observed conditions (with input from NO, NO2, O3, VOCs). It is useful to try other method (e.g. Brown et al., 2006) to derive N2O5 uptake coefficient. Brown, S. S., Ryerson, T. B., Wollny, A. G., Brock, C. A., Peltier, R., Sullivan, A. P., Weber, R. J., Dube, W. P., Trainer, M., Meagher, J. F., Fehsenfeld, F. C., and Ravishankara, A. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Nitrogen Oxides (Nox), Why and How They Are Controlled
United States Office of Air Quality EPA 456/F-99-006R Environmental Protection Planning and Standards November 1999 Agency Research Triangle Park, NC 27711 Air EPA-456/F-99-006R November 1999 Nitrogen Oxides (NOx), Why and How They Are Controlled Prepared by Clean Air Technology Center (MD-12) Information Transfer and Program Integration Division Office of Air Quality Planning and Standards U.S. Environmental Protection Agency Research Triangle Park, North Carolina 27711 DISCLAIMER This report has been reviewed by the Information Transfer and Program Integration Division of the Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents of this report reflect the views and policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products is not intended to constitute endorsement or recommendation for use. Copies of this report are available form the National Technical Information Service, U.S. Department of Commerce, 5285 Port Royal Road, Springfield, Virginia 22161, telephone number (800) 553-6847. CORRECTION NOTICE This document, EPA-456/F-99-006a, corrects errors found in the original document, EPA-456/F-99-006. These corrections are: Page 8, fourth paragraph: “Destruction or Recovery Efficiency” has been changed to “Destruction or Removal Efficiency;” Page 10, Method 2. Reducing Residence Time: This section has been rewritten to correct for an ambiguity in the original text. Page 20, Table 4. Added Selective Non-Catalytic Reduction (SNCR) to the table and added acronyms for other technologies. Page 29, last paragraph: This paragraph has been rewritten to correct an error in stating the configuration of a typical cogeneration facility. -

Chapter 10 100 Years of Progress in Gas-Phase Atmospheric Chemistry Research
CHAPTER 10 WALLINGTON ET AL. 10.1 Chapter 10 100 Years of Progress in Gas-Phase Atmospheric Chemistry Research T. J. WALLINGTON Research and Advanced Engineering, Ford Motor Company, Dearborn, Michigan J. H. SEINFELD California Institute of Technology, Pasadena, California J. R. BARKER Climate and Space Sciences and Engineering, University of Michigan, Ann Arbor, Michigan ABSTRACT Remarkable progress has occurred over the last 100 years in our understanding of atmospheric chemical composition, stratospheric and tropospheric chemistry, urban air pollution, acid rain, and the formation of airborne particles from gas-phase chemistry. Much of this progress was associated with the developing un- derstanding of the formation and role of ozone and of the oxides of nitrogen, NO and NO2, in the stratosphere and troposphere. The chemistry of the stratosphere, emerging from the pioneering work of Chapman in 1931, was followed by the discovery of catalytic ozone cycles, ozone destruction by chlorofluorocarbons, and the polar ozone holes, work honored by the 1995 Nobel Prize in Chemistry awarded to Crutzen, Rowland, and Molina. Foundations for the modern understanding of tropospheric chemistry were laid in the 1950s and 1960s, stimulated by the eye-stinging smog in Los Angeles. The importance of the hydroxyl (OH) radical and its relationship to the oxides of nitrogen (NO and NO2) emerged. The chemical processes leading to acid rain were elucidated. The atmosphere contains an immense number of gas-phase organic compounds, a result of emissions from plants and animals, natural and anthropogenic combustion processes, emissions from oceans, and from the atmospheric oxidation of organics emitted into the atmosphere. -

Chemical Bonding
Chemical Bonding Part Two: Covalent Bonds Electronegativity and Bond Character v Electronegativity - the relative ability of an atom to attract electrons in a chemical bond vThe greater the electronegativity difference, the stronger the bond is that forms between them vIf the electronegativity difference is: • Less than 0.5, then the bond is nonpolar covalent • Between 0.5 and 1.6, the bond is polar covalent • Greater than 1.6, then the bond is ionic Calculating Bond Character Electronegativity Determine the type of bond: Chart Carbon 2.55 1. H and O 3.44-2.20 = 1.2 Nitrogen 3.04 POLAR COVALENT Potassium 0.82 2. Ca and F 3.98-1.00 = 2.98 Fluorine 3.98 IONIC Hydrogen 2.20 Oxygen 3.44 3. Ba and O 3.44-0.89 = 2.55 Calcium 1.00 IONIC Chlorine 3.98 4. C and O 3.44-2.55 = 0.89 Barium 0.89 POLAR COVALENT Bromine 2.96 5. N and N 0-NONPOLAR COVALENT Covalent Bonds vCharacterized by a sharing of electrons vThe attraction of two atoms for a shared pair of electrons is called a covalent bond v In a covalent bond, atoms share electrons and neither atom has an ionic charge vA compound whose atoms are held together by covalent bonds is a covalent compound vMolecules are formed from covalent bonds Single and Multiple Covalent Bonds v When more than one pair of electrons are shared between atoms multiple covalent bonds form vThese can be double or triple bonds depending on the number of pairs of electrons shared Single and Multiple Covalent Bonds Characteristics of Covalent Bonds v Low melting points v Do not conduct electricity in any state v Solids are often soft or brittle v Many are gases at room temperature v Most are less soluble in water than ionic compounds and are not electrolytes Polar vs. -

Interferences in Photolytic NO2 Measurements: Explanation for An
Discussion Paper | Discussion Paper | Discussion Paper | Discussion Paper | Atmos. Chem. Phys. Discuss., 15, 28699–28747, 2015 www.atmos-chem-phys-discuss.net/15/28699/2015/ doi:10.5194/acpd-15-28699-2015 © Author(s) 2015. CC Attribution 3.0 License. This discussion paper is/has been under review for the journal Atmospheric Chemistry and Physics (ACP). Please refer to the corresponding final paper in ACP if available. Interferences in photolytic NO2 measurements: explanation for an apparent missing oxidant? C. Reed1, M. J. Evans1,2, P. Di Carlo3,4, J. D. Lee1,2, and L. J. Carpenter1 1Wolfson Atmospheric Chemistry Laboratories, Department of Chemistry, University of York, Heslington, York, YO10 5DD, UK 2NCAS, School of Earth and Environment, University of Leeds, Leeds, LS2 9JT, UK 3Centre of Excellence CEMTEPs, Universita’ degli studi di L’Aquila, Via Vetoio, 67010 Coppito, L’Aquila, Italy 4Dipartmento di Scienze Fisiche e Chimiche, Universita’ degli studi di L’Aquila, Via Vetoio, 67010 Coppito, L’Aquila, Italy Received: 6 October 2015 – Accepted: 7 October 2015 – Published: 23 October 2015 Correspondence to: J. D. Lee ([email protected]) Published by Copernicus Publications on behalf of the European Geosciences Union. 28699 Discussion Paper | Discussion Paper | Discussion Paper | Discussion Paper | Abstract Measurement of NO2 at low concentrations is non-trivial. A variety of techniques exist, with the conversion of NO2 into NO followed by chemiluminescent detection of NO be- ing prevalent. Historically this conversion has used a catalytic approach (Molybdenum); 5 however this has been plagued with interferences. More recently, photolytic conversion based on UV-LED irradiation of a reaction cell has been used. -

100 Most Important Chemical Compounds
64. Nitric Oxide 65. Nitrogen Dioxide 66. Nitrous Oxide CHEMICAL NAME = nitric monoxide nitrogen dioxide dinitrogen oxide CAS NUMBER = 10102–43–9 10102–44–0 10024–97–2 = MOLECULAR FORMULA NO NO2 N2O MOLAR MASS = 30.0 g/mol 46.0 g/mol 44.0 g/mol COMPOSITION = N(46.7%) O(53.3%) N(30.4%) O(69.6%) N(63.7%) MELTING POINT = −164°C −11.2 −90.8°C BOILING POINT = −152°C 21.1°C −88.6°C DENSITY = 1.35 g/L 2.05 g/L .99 g/L VAPOR DENSITY = 1.04 (air = 1) 1.58 1.53 Nitrogen oxides refer to several compounds composed of nitrogen and oxygen. Th is entry focuses on the three most common: nitric oxide, nitrogen dioxide, and nitrous oxide. Other nitrogen oxides not addressed include dinitrogen trioxide (N2O3), dinitrogen tetroxide (N2O4), nitrogen trioxide (NO3), and dinitrogen pentoxide (N2O5). As air pollutants, nitrogen oxides generally refer to the compounds nitric oxide and nitrogen dioxide. Nitrogen oxides exist as gaseous compounds under normal conditions, although nitrogen dioxide changes phases between liquid and gas near room temperature (its liquid density at 20°C is 1.45 g/cm3). Nitrogen monoxide (NO) is commonly called nitric oxide, and dinitrogen monoxide’s (N2O) common name is nitrous oxide. Nitric oxide and nitrogen dioxide are nonfl ammable, toxic gases. Nitric oxide is colorless and has a sharp sweet odor; nitrogen dioxide is a reddish-brown gas (or yellow liquid) with a strong, acrid odor. Nitrogen dioxide readily dimerizes to produce N2O4. Dinitrogen tetroxide production is favored as temperature decreases.