F1y3x SECTION VI PRODUCTS of the CHEMICAL OR
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Synthesis and Characterization of Nano Zinc Peroxide Photocatalyst for the Removal of Brilliant Green Dye from Textile Waste Water
International Journal of ChemTech Research CODEN (USA): IJCRGG, ISSN: 0974-4290, ISSN(Online):2455-9555 Vol.10 No.9, pp 477-486, 2017 Synthesis and characterization of nano zinc peroxide photocatalyst for the removal of brilliant green dye from textile waste water. Prashant L. Chaudhari*, Pallavi C. Kale Department of Chemical Engineering, BharatiVidyapeeth University College of Engineering, Pune, India Abstract : The zinc peroxide nanoparticles were synthesized by using oxidation-hydrolysis- precipitation process.With zinc acetate as a predecessor, Hydrogen peroxide used as a oxidizing agent and polyethylene glycol 200 (PEG 200) was used as a surface modifier. Characterization of zinc peroxide nanoparticles was done by X-ray diffraction [XRD], Fourier transformer infra red [FTIR] and transmission electron microscope [TEM]. The parameters like pH, dye concentration, dosage of nanoparticle catalyst, temperature and contact time were studied for the application of Zinc peroxide nanopaticle as a catalyst for removal of a Brilliant green dye from synthetic sample. Excellent degradation efficiency of brilliant green dye was 86.68% achieved by zinc peroxide with PEG as a catalyst and 84.16% achieved by zinc peroxide without PEG as a catalyst at 120 min of photo catalytic reaction. The maximum degradation efficiency of brilliant green dye (86.68%) was achieved at the optimum operational conditions: initial concentration of dye 9mg/l, catalyst dosage of 200 mg, pH of solution will be in between 6-7. Keywords : Zinc peroxide nanoparticles, Brilliant green dye, Zinc oxide, Textile waste. Introduction The consumption of dyes was highly increased by textile industries in recent years and because of this scenario large amount of coloured wastewater from such industries create serious problems to the environment. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0027386 A1 Kurihara Et Al
US 20110027386A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0027386 A1 Kurihara et al. (43) Pub. Date: Feb. 3, 2011 (54) ANTMICROBAL. ZEOLITE AND (30) Foreign Application Priority Data ANTMICROBAL COMPOSITION Feb. 22, 2006 (JP) ................................. 2006-045241 (75) Inventors: Yasuo Kurihara, Nagoya-shi (JP); Kumiko Miyake, Nagoya-shi (JP); Publication Classification Masashi Uchida, Nagoya-shi (JP) (51) Int. Cl. Correspondence Address: AOIN 59/6 (2006.01) NIXON & VANDERHYE, PC COB 39/02 (2006.01) 901 NORTH GLEBE ROAD, 11TH FLOOR AOIP I/00 (2006.01) ARLINGTON, VA 22203 (US) (52) U.S. Cl. .......................... 424/618; 423/701; 423/700 (73) Assignee: Sinanen Zeomic Co., Ltd., (57) ABSTRACT Nagoya-Shi (JP) The present invention relates to antimicrobial zeolite which comprises zeolite whereina hardly soluble zinc salt is formed (21) Appl. No.: 12/923,854 within fine pores present therein and an antimicrobial com position which comprises the foregoing antimicrobial Zeolite (22) Filed: Oct. 12, 2010 in an amount ranging from 0.05 to 80% by mass. The antimi crobial Zeolite according to the present invention can widely Related U.S. Application Data be applied, without causing any color change, even to the (63) Continuation of application No. 1 1/705,460, filed on goods which undergo color changes with the elapse of time Feb. 13, 2007. when the conventional antimicrobial zeolite is added. US 2011/002738.6 A1 Feb. 3, 2011 ANTMICROBAL. ZEOLITE AND 3. An antimicrobial composition comprising the foregoing ANTMICROBAL COMPOSITION antimicrobial zeolite as set forth in the foregoing item 1 or 2 in an amount ranging from 0.05 to 80% by mass. -
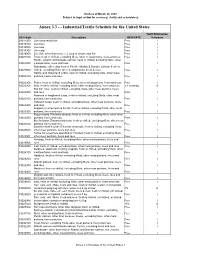
US Schedule for Internet V2
Draft as of March 23, 2007 Subject to legal review for accuracy, clarity and consistency. Annex 3.3 - - Industrial/Textile Schedule for the United States Tariff Elimination US 8 digit Description MFN RATE Schedule 03011000 Live ornamental fish Free I 03019100 Live trout Free I 03019200 Live eels Free I 03019300 Live carp Free I 03019900 Live fish, other than trout, eel, carp or ornamental fish Free I 03021100 Trout, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I Pacific, Atlantic and Danube salmon, fresh or chilled, excluding fillets, other 03021200 meat portions, livers and roes Free I Salmonidae other than trout or Pacific, Atlantic & Danube salmon, fresh or 03021900 chilled, excluding fillets, other meat portions, livers & roes Free I Halibut and Greenland turbot, fresh or chilled, excluding fillets, other meat 03022100 portions, livers and roes Free I 03022200 Plaice, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I 03022300 Sole, fresh or chilled, excluding fillets, other meat portions, livers and roes 1.1 cents/kg A Flat fish, nesi, fresh or chilled, excluding fillets, other meat portions, livers 03022900 and roes Free I Albacore or longfinned tunas, fresh or chilled, excluding fillets, other meat 03023100 portions, livers and roes Free I Yellowfin tunas, fresh or chilled, excluding fillets, other meat portions, livers 03023200 and roes Free I Skipjack or stripe-bellied bonito, fresh or chilled, excluding fillets, other meat 03023300 portions, livers and roes Free -

Study of Phosphorus Behaviour in Levitated Silicon-Iron Droplets
STUDY OF PHOSPHORUS BEHAVIOUR IN LEVITATED SILICON-IRON DROPLETS by Katherine Le A thesis submitted in conformity with the requirements for the degree of Master of Applied Science Department of Materials Science and Engineering University of Toronto © Copyright by Katherine Le 2016 ii Study of Phosphorus Behaviour in Levitated Si-Fe Droplets Katherine Le Master of Applied Science Department of Materials Science and Engineering University of Toronto 2016 Abstract While the treatment of relatively inexpensive ferrosilicon alloys is a potential refining route in order to generate solar grade silicon, phosphorus is one of the more difficult impurities to remove by conventional processing. In this project, electromagnetic levitation was used to investigate the dephosphorization of ferrosilicon alloy droplets exposed to H2-Ar gas mixtures under various experimental conditions including, refining time, temperature (1450°C-1720°C), H2-Ar gas concentrations and flow rate, iron alloying content, and initial phosphorus concentration. Reaction rates increased with higher refining times, temperatures, and H2 gas concentrations. With unknown parameters associated with the kinetics of gas phase reactions, the approach involved comparison of apparent activation energies derived for the chemical reaction and gas diffusion steps of the dephosphorization process. The phosphorus removal rate is thought to be controlled by the interfacial reaction step; further work is required to confirm this conclusion. iii Acknowledgements I would like to express my gratitude and respect to my supervisor, Prof. Alex McLean for the opportunity to work on this research. I am thankful for his guidance, wisdom and encouragement throughout the course of my studies. He is a truly inspiring person, and a great enabler of new learning opportunities. -

Binary and Ternary Transition-Metal Phosphides As Hydrodenitrogenation Catalysts
Research Collection Doctoral Thesis Binary and ternary transition-metal phosphides as hydrodenitrogenation catalysts Author(s): Stinner, Christoph Publication Date: 2001 Permanent Link: https://doi.org/10.3929/ethz-a-004378279 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss. ETH No. 14422 Binary and Ternary Transition-Metal Phosphides as Hydrodenitrogenation Catalysts A dissertation submitted to the Swiss Federal Institute of Technology Zurich for the degree of Doctor of Natural Sciences Presented by Christoph Stinner Dipl.-Chem. University of Bonn born February 27, 1969 in Troisdorf (NRW), Germany Accepted on the recommendation of Prof. Dr. Roel Prins, examiner Prof. Dr. Reinhard Nesper, co-examiner Dr. Thomas Weber, co-examiner Zurich 2001 I Contents Zusammenfassung V Abstract IX 1 Introduction 1 1.1 Motivation 1 1.2 Phosphides 4 1.2.1 General 4 1.2.2 Classification 4 1.2.3 Preparation 5 1.2.4 Properties 12 1.2.5 Applications and Uses 13 1.3 Scope of the Thesis 14 1.4 References 16 2 Characterization Methods 1 2.1 FT Raman Spectroscopy 21 2.2 Thermogravimetric Analysis 24 2.3 Temperature-Programmed Reduction 25 2.4 X-Ray Powder Diffractometry 26 2.5 Nitrogen Adsorption 28 2.6 Solid State Nuclear Magnetic Resonance Spectroscopy 28 2.7 Catalytic Test 33 2.8 References 36 3 Formation, Structure, and HDN Activity of Unsupported Molybdenum Phosphide 37 3.1 Introduction -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Zinc Oxide—From Synthesis to Application: a Review
Materials 2014, 7, 2833-2881; doi:10.3390/ma7042833 OPEN ACCESS materials ISSN 1996-1944 www.mdpi.com/journal/materials Review Zinc Oxide—From Synthesis to Application: A Review Agnieszka Kołodziejczak-Radzimska * and Teofil Jesionowski Institute of Chemical Technology and Engineering, Faculty of Chemical Technology, Poznan University of Technology, M. Sklodowskiej-Curie 2, PL-60965 Poznan, Poland; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +48-61-665-3626; Fax: +48-61-665-3649. Received: 17 December 2013; in revised form; 25 March 2014 / Accepted: 27 March 2014 / Published: 9 April 2014 Abstract: Zinc oxide can be called a multifunctional material thanks to its unique physical and chemical properties. The first part of this paper presents the most important methods of preparation of ZnO divided into metallurgical and chemical methods. The mechanochemical process, controlled precipitation, sol-gel method, solvothermal and hydrothermal method, method using emulsion and microemulsion enviroment and other methods of obtaining zinc oxide were classified as chemical methods. In the next part of this review, the modification methods of ZnO were characterized. The modification with organic (carboxylic acid, silanes) and inroganic (metal oxides) compounds, and polymer matrices were mainly described. Finally, we present possible applications in various branches of industry: rubber, pharmaceutical, cosmetics, textile, electronic and electrotechnology, photocatalysis were introduced. This review provides useful information for specialist dealings with zinc oxide. Keywords: zinc oxide; synthesis; modification; application 1. Introduction Zinc oxide, with its unique physical and chemical properties, such as high chemical stability, high electrochemical coupling coefficient, broad range of radiation absorption and high photostability, is a multifunctional material [1,2]. -

Download SCOPE Newsletter
With participation of the European Commission: DG GROW and Joint Research Centre Summary of joint European Commission – ESPP webinar th on P4 (phosphorus) Critical Raw Material, 9 July 2020 This ESPP SCOPE Newsletter summarises discussions at the expert webinar jointly organised by ESPP and the European Commission on 9th July, with participation of Contents the leading companies using phosphorus (P4) or its derivatives in Europe, with additional input from the The EU Critical Raw Materials: “Phosphate Rock” and “Phosphorus”........................................................................... 2 independent industry expert Willem Schipper. The content (Fig. i) Overview numbers: from phosphate rock to P4 / P4-derivatives below has been seen by the participants but does not and applications .................................................................................. 3 represent their position, it represents ESPP’s own How is P4 produced? the phosphorus furnace .............. 4 assessment. Photo: P4 furnace ................................................................................ 4 Schematic: P4 manufacture process (simplified) ................................. 4 This webinar aimed to provide expert input to the European Which chemicals critically depend on P4 / P4 derivatives? . 5 Commission’s MSA (Material System Analysis) of the EU dependency on P4 concerns many industry sectors . 5 specific industrial form of “Phosphorus”, P4, as a Critical (Fig. ii) Production via wet-acid or via P4 / P4 derivatives / ‘thermal’ Raw -

Zinc Oxide: New Insights Into a Material for All Ages
ZINC OXIDE: NEW INSIGHTS INTO A MATERIAL FOR ALL AGES BY AMIR MOEZZI BSc. Chemical Engineering A Dissertation Submitted for the Degree of Doctor of Philosophy University of Technology Sydney 2012 Certificate of authorship/originality I certify that the work in this thesis has not previously been submitted for a degree nor has it been submitted as part of requirements for a degree except as fully acknowledged within the text. I also certify that the thesis has been written by me. Any help that I have received in my research work and the preparation of the thesis itself has been acknowledged. In addition, I certify that all information sources and literature used are indicated in the thesis. Amir Moezzi 11/10/2012 Copyright © 2012 by Amir Moezzi All rights reserved. No part of this publication may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without the prior written permission of the author, except in the case of brief quotations embodied in critical reviews and certain other non- commercial uses permitted by copyright law. Printed and bound in Australia ii Acknowledgements I would like to express my special appreciation to the company PT. Indo Lysaght, of Indonesia, for financial support for my project and very kind hospitality during the visits from their chemical plants. My greatest debts are to my principal supervisor, Prof. Michael Cortie, and my co- supervisors Dr Andrew McDonagh, who gave me the opportunity to undertake this project and for their support and encouragement throughout the project. -

Catalog of Standard Reference Materials
NATL INST. OF STAND & TECH Bureau of Standards £-01 Admin. Bldg. AUG 1 8 1970 NBS SPECIAL PUBLICATION 260 NBS JULY 1970 EDITION PUBLICATIONS Catalog of 9TAMDARD "REFERENCE MATERIALS U.S. DEPARTMENT OF COMMERCE National Bureau of Standards - NATIONAL BUREAU OF STANDARDS The National Bureau of Standards 1 was established by an act of Congress March 3, 1901. Today, in addition to serving as the Nation's central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To this end the Bureau conducts research and provides central national services in four broad program areas. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Radiation Research, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and com- merce. The Institute consists of an Office of Measurement Services and the following technical divisions: Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic and Molec- ular Physics—Radio Physics - —Radio Engineering -—Time and Frequency -—Astro- physics - —Cryogenics. -

Other Than Radioactive Ores) Answering to a Description in Heading 2844 Or 2845 Are to Be Classified in Those Headings and in No Other Heading of the Tariff Schedule
)&f1y3X SECTION VI PRODUCTS OF THE CHEMICAL OR ALLIED INDUSTRIES VI-1 Notes 1. (a) Goods (other than radioactive ores) answering to a description in heading 2844 or 2845 are to be classified in those headings and in no other heading of the tariff schedule. (b) Subject to paragraph (a) above, goods answering to a description in heading 2843 or 2846 are to be classified in those headings and in no other heading of this section. 2. Subject to note 1 above, goods classifiable in heading 3004, 3005, 3006, 3212, 3303, 3304, 3305, 3306, 3307, 3506, 3707 or 3808 by reason of being put up in measured doses or for retail sale are to be classified in those headings and in no other heading of the tariff schedule. 3. Goods put up in sets consisting of two or more separate constituents, some or all of which fall in this section and are intended to be mixed together to obtain a product of section VI or VII, are to be classified in the heading appropriate to that product, provided that the constituents are: (a) Having regard to the manner in which they are put up, clearly identifiable as being intended to be used together without first being repacked; (b) Entered together; and (c) Identifiable, whether by their nature or by the relative proportions in which they are present, as being complementary one to another. Additional U.S. Notes 1. In determining the amount of duty applicable to a solution of a single compound in water subject to duty in this section at a specific rate, an allowance in weight or volume, as the case may be, shall be made for the water in excess of any water of crystallization which may be present in the undissolved compound. -

Minerals, Critical Minerals
MINERALS, CRITICAL MINERALS, AND THE U.S. ECONOMY Prepublication Version THIS PREPUBLICATION VERSION OF MINERALS, CRITICAL MINERALS, AND THE U.S. ECONOMY has been provided to the public to facilitate timely access to the committee’s findings. Although the substance of the report is final, editorial changes may be made throughout the text, and citations will be checked prior to publication. The final report will be available through the National Academies Press in the December/January timeframe. MINERALS, CRITICAL MINERALS, AND THE U.S. ECONOMY Committee on Critical Mineral Impacts on the U.S. Economy Committee on Earth Resources Board on Earth Sciences and Resources Division on Earth and Life Studies THE NATIONAL ACADEMIES PRESS Washington, D.C. www.nap.edu Prepublication Version – Subject to Further Editorial Revision THE NATIONAL ACADEMIES PRESS • 500 Fifth Street, N.W. • Washington, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Institute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance. This study was supported by the Department of the Interior / U.S. Geological Survey, under Award No. 06HQGR0204, and by the National Mining Association. The opinions, findings, and conclusions or recommendations contained in this document are those of the authors and do not necessarily reflect the views of the organizations or agencies that provided support for the project.