Download SCOPE Newsletter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Eastwood)\WPD Files\MSDS Easthill E6271CT Chassis B
EW #11176ZP - Extreme Chassis Black Satin Quart Page 1 of 6 MATERIAL SAFETY Chassis Black High Gloss Part No. E6175CT11176ZP Liquid DATA SHEET Revision 1 ˜ February 6, 2006 EMERGENCY OVERVIEW FLAMMABLE LIQUID. AVOID CONTACT WITH SKIN AND EYES. VAPOR HARMFUL. INTENTIONAL MISUSE BY DELIBERATELY CONCENTRATING AND INHALING THE CONTENTS MAY BE HARMFUL OR FATAL. INGESTION MAY BE HARMFUL OR FATAL. SECTION 1 CHEMICAL PRODUCT AND COMPANY IDENTIFICATION MANUFACTURER:MANUFACTURED FOR: Chem-Pak,The Easthill Inc.Group SUPPLIER: The Easthill Group, Inc. dba/ The Eastwood Company 242263 CorningShoemaker Way Road 263 Shoemaker Road Martinsburg,Pottstown, PA WV 19464 25401 USA Pottstown PA 19464 USA USA & Canada: 800-345-1178 INFORMATION: 800-336-9828Outside USA: (610) 323-2200 INFORMATION: 610-323-2200 EMERGENCY: 800-255-3924Chem-Trec 800-424-9300 (24 hr) Chem-Tel EMERGENCY: 1-800-424-9300 PRODUCT IDENTIFIER: 11176ZPE6175CT, Gallon SUPPLIER NUMBER: 11177ZP 11177ZPE6176CT, Quart 11176ZP PRODUCT DESCRIPTION: Extreme Chassis Black PRODUCT TYPE: Liquid REVISION NUMBER: 1 CAS NUMBER: Mixture REVISION DATE: January 30, 2006 EMAIL: [email protected] PRINT DATE: February 6, 2006 WEBSITE: http://www.chem-pak.com SECTION 2 COMPOSITION / INFORMATION ON INGREDIENTS INGREDIENT CAS NUMBER OSHA PEL NIOSH REL ACGIH PEL IDLH % WT Xylene 001330-20-7 100 ppm 100 ppm 100 ppm 900 ppm 20-30 n-Hexane 000110-54-2 500 ppm 50 ppm 50 ppm 1100 ppm 20-30 Toluene 000108-88-3 200 ppm 100 ppm 50 ppm 500 ppm < 10 Ethyl Benzene 000100-41-4 100 ppm 100 ppm 100 ppm 800 ppm < 10 Butyl Acetate 000123-86-4 150 ppm 150 ppm 150 ppm 1700 ppm < 10 Proprietary Resin Trade Secret N/E N/E N/E N/E < 10 Carbon Black 001333-86-4 3.5 mg/m3 3.5 mg/m3 3.5 mg/m3 1750 mg/m3 < 10 Methyl Alcohol 000067-56-1 200 ppm 200 ppm 200 ppm 6000 ppm < 10 See Section 11 for LD50 and LC50 Species/Route Information. -

Transition Metal Phosphides for the Catalytic Hydrodeoxygenation of Waste Oils Into Green Diesel
catalysts Review Transition Metal Phosphides for the Catalytic Hydrodeoxygenation of Waste Oils into Green Diesel M. Consuelo Alvarez-Galvan * , Jose M. Campos-Martin * and Jose L. G. Fierro * Energy and Sustainable Chemistry Group (EQS), Instituto de Catálisis y Petroleoquímica, CSIC, c/Marie Curie, 2 Cantoblanco, 28049 Madrid, Spain * Correspondence: [email protected] (M.C.A.-G.); [email protected] (J.M.C.-M.); jlgfi[email protected] (J.L.G.F.) Received: 28 February 2019; Accepted: 15 March 2019; Published: 22 March 2019 Abstract: Recently, catalysts based on transition metal phosphides (TMPs) have attracted increasing interest for their use in hydrodeoxygenation (HDO) processes destined to synthesize biofuels (green or renewable diesel) from waste vegetable oils and fats (known as hydrotreated vegetable oils (HVO)), or from bio-oils. This fossil-free diesel product is produced completely from renewable raw materials with exceptional quality. These efficient HDO catalysts present electronic properties similar to noble metals, are cost-efficient, and are more stable and resistant to the presence of water than other classical catalytic formulations used for hydrotreatment reactions based on transition metal sulfides, but they do not require the continuous supply of a sulfide source. TMPs develop a bifunctional character (metallic and acidic) and present tunable catalytic properties related to the metal type, phosphorous-metal ratio, support nature, texture properties, and so on. Here, the recent progress in TMP-based catalysts for HDO of waste oils is reviewed. First, the use of TMPs in catalysis is addressed; then, the general aspects of green diesel (from bio-oils or from waste vegetable oils and fats) production by HDO of nonedible oil compounds are presented; and, finally, we attempt to describe the main advances in the development of catalysts based on TMPs for HDO, with an emphasis on the influence of the nature of active phases and effects of phosphorous, promoters, and preparation methods on reactivity. -

Black Phosphorus As a New Lubricant
Friction 6(1): 116–142 (2018) ISSN 2223-7690 https://doi.org/10.1007/s40544-018-0204-z CN 10-1237/TH RESEARCH ARTICLE Black phosphorus as a new lubricant Wei WANG, Guoxin XIE*, Jianbin LUO* State Key Laboratory of Tribology, Tsinghua University, Beijing 100084, China Received: 20 June 2017 / Revised: 18 October 2017 / Accepted: 07 December 2017 © The author(s) 2018. This article is published with open access at Springerlink.com Abstract: In recent years, a new 2D-layered material—black phosphorus (BP)—has been a rising star after the era of graphene owing to its high charge carrier mobility, tunable direct bandgap and unique in-plane anisotropic structure. With the development of the synthesis and modification methods of BP, its extensive applications, e.g., transistors, batteries and optoelectronics have emerged. In order to explore its full potential, research into the tribological properties of BP 2D-layered materials such as lubrication additives and fillers in self-lubricating composite materials would be not only of high scientific value but also of practical significance. In this work, recent advances on the friction and lubrication properties of BP nanosheets made by our group, including the micro-friction properties, the lubrication properties of BP nanosheets as water-based and oil-based lubrication additives, and the friction and wear of BP/PVDF composites will be presented. Finally, the future challenges and opportunities in the use of BP materials as lubricants will be discussed. Keywords: black phosphorus; two-dimensional (2D) material; lubricant additive; self-lubricating composite materials; friction 1 Introduction certain period owing to the finite lubricant thickness, and it can be easily affected by the environment. -

Microplastics in Shore Sediments – Development of a Suitable Extraction and Purification Technique for Citizen Science Projects
Christian Pacher, BSc Microplastics in shore sediments – Development of a suitable extraction and purification technique for citizen science projects Masterarbeit zur Erlangung des akademischen Grades eines Master of Science der Studienrichtung Ökologie und Evolutionsbiologie an der Karl-Franzens-Universität Graz Betreuer: Priv.-Doz. Mag. Dr.rer.nat. Stephan Koblmüller Institut für Biologie Abstract ..................................................................................................................................... 4 Introduction .............................................................................................................................. 5 Plastic – Our omnipresent companion .................................................................................... 5 Dangers of microplastics ........................................................................................................ 6 Plastic distribution in the Mediterranean Sea ......................................................................... 8 Microplastics in shore sediments ............................................................................................ 9 Citizen science ...................................................................................................................... 10 Location of the sampling spot .............................................................................................. 11 Material and methods ........................................................................................................... -
US Schedule for Internet V2
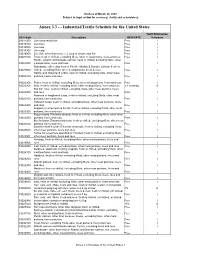
Draft as of March 23, 2007 Subject to legal review for accuracy, clarity and consistency. Annex 3.3 - - Industrial/Textile Schedule for the United States Tariff Elimination US 8 digit Description MFN RATE Schedule 03011000 Live ornamental fish Free I 03019100 Live trout Free I 03019200 Live eels Free I 03019300 Live carp Free I 03019900 Live fish, other than trout, eel, carp or ornamental fish Free I 03021100 Trout, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I Pacific, Atlantic and Danube salmon, fresh or chilled, excluding fillets, other 03021200 meat portions, livers and roes Free I Salmonidae other than trout or Pacific, Atlantic & Danube salmon, fresh or 03021900 chilled, excluding fillets, other meat portions, livers & roes Free I Halibut and Greenland turbot, fresh or chilled, excluding fillets, other meat 03022100 portions, livers and roes Free I 03022200 Plaice, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I 03022300 Sole, fresh or chilled, excluding fillets, other meat portions, livers and roes 1.1 cents/kg A Flat fish, nesi, fresh or chilled, excluding fillets, other meat portions, livers 03022900 and roes Free I Albacore or longfinned tunas, fresh or chilled, excluding fillets, other meat 03023100 portions, livers and roes Free I Yellowfin tunas, fresh or chilled, excluding fillets, other meat portions, livers 03023200 and roes Free I Skipjack or stripe-bellied bonito, fresh or chilled, excluding fillets, other meat 03023300 portions, livers and roes Free -

Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life
Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life Item Type text; Electronic Dissertation Authors Pasek, Matthew Adam Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 07/10/2021 06:16:37 Link to Item http://hdl.handle.net/10150/194288 PHOSPHORUS AND SULFUR COSMOCHEMISTRY: IMPLICATIONS FOR THE ORIGINS OF LIFE by Matthew Adam Pasek ________________________ A Dissertation Submitted to the Faculty of the DEPARTMENT OF PLANETARY SCIENCE In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College UNIVERSITY OF ARIZONA 2 0 0 6 2 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Matthew Adam Pasek entitled Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy _______________________________________________________________________ Date: 04/11/2006 Dante Lauretta _______________________________________________________________________ Date: 04/11/2006 Timothy Swindle _______________________________________________________________________ Date: 04/11/2006 -

Study of Phosphorus Behaviour in Levitated Silicon-Iron Droplets
STUDY OF PHOSPHORUS BEHAVIOUR IN LEVITATED SILICON-IRON DROPLETS by Katherine Le A thesis submitted in conformity with the requirements for the degree of Master of Applied Science Department of Materials Science and Engineering University of Toronto © Copyright by Katherine Le 2016 ii Study of Phosphorus Behaviour in Levitated Si-Fe Droplets Katherine Le Master of Applied Science Department of Materials Science and Engineering University of Toronto 2016 Abstract While the treatment of relatively inexpensive ferrosilicon alloys is a potential refining route in order to generate solar grade silicon, phosphorus is one of the more difficult impurities to remove by conventional processing. In this project, electromagnetic levitation was used to investigate the dephosphorization of ferrosilicon alloy droplets exposed to H2-Ar gas mixtures under various experimental conditions including, refining time, temperature (1450°C-1720°C), H2-Ar gas concentrations and flow rate, iron alloying content, and initial phosphorus concentration. Reaction rates increased with higher refining times, temperatures, and H2 gas concentrations. With unknown parameters associated with the kinetics of gas phase reactions, the approach involved comparison of apparent activation energies derived for the chemical reaction and gas diffusion steps of the dephosphorization process. The phosphorus removal rate is thought to be controlled by the interfacial reaction step; further work is required to confirm this conclusion. iii Acknowledgements I would like to express my gratitude and respect to my supervisor, Prof. Alex McLean for the opportunity to work on this research. I am thankful for his guidance, wisdom and encouragement throughout the course of my studies. He is a truly inspiring person, and a great enabler of new learning opportunities. -

Binary and Ternary Transition-Metal Phosphides As Hydrodenitrogenation Catalysts
Research Collection Doctoral Thesis Binary and ternary transition-metal phosphides as hydrodenitrogenation catalysts Author(s): Stinner, Christoph Publication Date: 2001 Permanent Link: https://doi.org/10.3929/ethz-a-004378279 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss. ETH No. 14422 Binary and Ternary Transition-Metal Phosphides as Hydrodenitrogenation Catalysts A dissertation submitted to the Swiss Federal Institute of Technology Zurich for the degree of Doctor of Natural Sciences Presented by Christoph Stinner Dipl.-Chem. University of Bonn born February 27, 1969 in Troisdorf (NRW), Germany Accepted on the recommendation of Prof. Dr. Roel Prins, examiner Prof. Dr. Reinhard Nesper, co-examiner Dr. Thomas Weber, co-examiner Zurich 2001 I Contents Zusammenfassung V Abstract IX 1 Introduction 1 1.1 Motivation 1 1.2 Phosphides 4 1.2.1 General 4 1.2.2 Classification 4 1.2.3 Preparation 5 1.2.4 Properties 12 1.2.5 Applications and Uses 13 1.3 Scope of the Thesis 14 1.4 References 16 2 Characterization Methods 1 2.1 FT Raman Spectroscopy 21 2.2 Thermogravimetric Analysis 24 2.3 Temperature-Programmed Reduction 25 2.4 X-Ray Powder Diffractometry 26 2.5 Nitrogen Adsorption 28 2.6 Solid State Nuclear Magnetic Resonance Spectroscopy 28 2.7 Catalytic Test 33 2.8 References 36 3 Formation, Structure, and HDN Activity of Unsupported Molybdenum Phosphide 37 3.1 Introduction -

(Eastwood)\WPD Files\MSDS Easthill E6271CT Chassis B
EW #10043 ZP - EW Chassis Black Satin Quarts Page 1 of 6 MATERIAL SAFETY Chassis Black Satin Part No. E6271CT10043ZP Liquid DATA SHEET Revision 1 ˜ February 6, 2006 EMERGENCY OVERVIEW FLAMMABLE LIQUID. AVOID CONTACT WITH SKIN AND EYES. VAPOR HARMFUL. INTENTIONAL MISUSE BY DELIBERATELY CONCENTRATING AND INHALING THE CONTENTS MAY BE HARMFUL OR FATAL. INGESTION MAY BE HARMFUL OR FATAL. SECTION 1 CHEMICAL PRODUCT AND COMPANY IDENTIFICATION The Easthill Group MANUFACTUREDMANUFACTURER: FOR: dba/Chem-Pak, The Eastwood Inc. Co. SUPPLIER: The Easthill Group, Inc. 242263 CorningShoemaker Way Rd. 263 Shoemaker Road Martinsburg,Pottstown, PA WV 19464 25401 USA Pottstown PA 19464 USA USA and Canada: 1-800-345-1178 INFORMATION: 800-336-9828Outside USA: (610) 323-2200 INFORMATION: 610-323-2200 EMERGENCY: 800-255-3924Chem-Trec 1-800-424-9300 (24 hr) Chem-Tel EMERGENCY: 1-800-424-9300 PRODUCT IDENTIFIER: E6271CT,10043ZP Quart SUPPLIER NUMBER: 10043ZP PRODUCT DESCRIPTION: Chassis Black Satin PRODUCT TYPE: Liquid REVISION NUMBER: 1 CAS NUMBER: Mixture REVISION DATE: February 6, 2006 EMAIL: [email protected] PRINT DATE: February 6, 2006 WEBSITE: http://www.chem-pak.com SECTION 2 COMPOSITION / INFORMATION ON INGREDIENTS INGREDIENT CAS NUMBER OSHA PEL NIOSH REL ACGIH PEL IDLH % WT Toluene 000108-88-3 200 ppm 100 ppm 50 ppm 500 ppm 20-30 Xylene 001330-20-7 100 ppm 100 ppm 100 ppm 900 ppm 20-30 Acetone 000067-64-1 1000 ppm 250 ppm 500 ppm 750 ppm < 10 Stoddard Solvent 008052-41-3 500 ppm 350 mg/m3 100 ppm 20 g/m3 < 10 Methyl Alcohol 000067-56-1 200 ppm 200 ppm 200 ppm 6000 ppm < 10 Ethyl Benzene 000100-41-4 100 ppm 100 ppm 100 ppm 800 ppm < 10 Carbon Black 001333-86-4 3.5 mg/m3 3.5 mg/m3 3.5 mg/m3 1750 mg/m3 < 10 See Section 11 for LD50 and LC50 Species/Route Information. -

Hazard Symbols and Their Meanings Pdf
Hazard symbols and their meanings pdf Continue The symbols of danger came far from the rudimentary drawings used to refer to the poison in the early 1800s. As a result of the updated requirements for chemical labeling, OSHA 2016 marks the first full year of the adoption of the Globally Agreed Chemical Classification and Labeling System (GHS) in the United States. The GHS system, which includes the OSHA (HCS) OSHA Threat Communication Standard, consists of nine characters, or pictograms, that recognize the hazards associated with certain substances. The use of eight out of nine are mandatory in the U.S., with the exception of an environmental pictogram (see below). Each pictogram covers a certain type of hazard and is designed to be immediately recognizable to anyone who processes hazardous materials. In addition to pictograms, labels should include a signal word (danger or warning), a brief statement of danger, and a statement outlining ways to prevent exposure. Pictograms and health hazard descriptions: a cancer- causing agent (carcinogen) or a substance with respiratory, reproductive or organ toxicity that over time causes damage (chronic or long-term health hazard). Flames: flammable materials or substances to self-ignite when exposed to water or air (pyrophor), or which emit flammable gas. exclamation point: Immediate skin, eyes or respiratory tract irritant, or narcotic. Gas cylinder: Gas stored under pressure, such as ammonia or liquid nitrogen. Corrosion: Materials that cause skin corrosion/burns or eye damage on contact, or are corrosive to metals. Exploding bomb: Explosives, including organic peroxides and highly unstable material, run the risk of exploding even without exposure to air (self-activity). -

TS Lab Safety Rules for Chemistry Labs (Version December 2020)
20201106-tslab-chemistry-lab-safety-rules-instruction-short TS Lab Safety Rules for Chemistry Labs (version December 2020) This is just a compendium of some essential issues concerning the safety in a chemistry lab. For detailed information and issues that are not listed you are legally bound to inform yourself about “Working Safely in Laboratories - Basic Principles and Guidelines” DGUV Information 213-851 (https://www.sicherheitswesen.verwaltung.uni-muenchen.de/arbeitssicherheit/infos/index.html). You are also required to read and approve our lab's online safety webpage at https://thornseshold.cup.uni-muenchen.de/resources/safety/ General Rules • Smoking in the lab is prohibited. • Eating and drinking in the lab is prohibited. Food and drink must not be brought into the lab! • Chemicals must not be stored in containers that lead to confusion with food or beverages! • All containers have to be labelled according to CLP regulation (DGUV Information 213-851). • You have to wear protective eye in the lab! • You have to wear adequate protective clothes such as lab coats and slip-resistant footwear in the lab! Contaminated lab coats have to be changed. Wear lab coats only in lab-like workspaces! Whenever you enter libraries, lecture halls, seminar rooms, dining halls, or social rooms put them off! • If you wear protective gloves remove them when leaving the lab! Do not touch any device that will lead to contamination of your colleagues! • Do not work in labs with a red light display! Do not work in a hood with a red lit flow indicator! • All actions with hazards (e.g. -

Download (4Mb)
Original citation: Zhenqing, Li, He, Chaoyu, Ouyang, Tao, Zhang, Chunxiao, Tang, Chao, Roemer, Rudolf A. and Zhong, Jianxin (2018) New allotropes of phosphorene with remarkable stability and intrinsic piezoelectricity. Physical Review Applied, 9 . 044032. doi:10.1103/PhysRevApplied.9.044032 Permanent WRAP URL: http://wrap.warwick.ac.uk/101768 Copyright and reuse: The Warwick Research Archive Portal (WRAP) makes this work by researchers of the University of Warwick available open access under the following conditions. Copyright © and all moral rights to the version of the paper presented here belong to the individual author(s) and/or other copyright owners. To the extent reasonable and practicable the material made available in WRAP has been checked for eligibility before being made available. Copies of full items can be used for personal research or study, educational, or not-for-profit purposes without prior permission or charge. Provided that the authors, title and full bibliographic details are credited, a hyperlink and/or URL is given for the original metadata page and the content is not changed in any way. Publisher statement: © 2018 American Physical Society Published version: https://doi.org/10.1103/PhysRevApplied.9.044032 A note on versions: The version presented here may differ from the published version or, version of record, if you wish to cite this item you are advised to consult the publisher’s version. Please see the ‘permanent WRAP url’ above for details on accessing the published version and note that access may require a subscription. For more information, please contact the WRAP Team at: [email protected] warwick.ac.uk/lib-publications New allotropes of phosphorene with remarkable stability and intrinsic piezoelectricity Zhenqing Li,1, 2 Chaoyu He,1, 2, ∗ Tao Ouyang,1, 2 Chunxiao Zhang,1, 2 Chao Tang,1, 2, y Rudolf A.