Catalog of Standard Reference Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Silver Steel Data Sheet
SSS SABEN SILVER STEEL 1.2210 PRECISION GROUND DOWELL ROD B.S.1407 WERKSTOFF No C Si Mn Cr Mo W V Co 1.20 0.40 0.40 Features and Uses Tempering Applications include Temper according to the Saben Silver Steel is bright screwdrivers, punches, shafts, purpose for which the parts are finished rod produced from hot axles, pinions, pins, die posts, required generally between rolled bar by means of instrument parts, model parts, 150 / 300ºC centreless grinding. taps and drills for mild steel, The high carbon content of this engravers tools, and fine Rockwell C Temperature steel means that it can be cutters. 63/65 as quenched hardened to give considerable 63/65 120ºC wear resistance and the Hardening 64/62 150ºC chromium content adds to the 62/61 200ºC strength and hardenability It is preferable to heat the tools 59/58 250ºC As supplied however, the steel in a controlled atmosphere. If 56/55 300ºC is machinable owing to the this is not possible, pack 54/53 350ºC annealing treatment given to it hardening is recommended. A 50/48 400ºC prior to grinding. reducing atmosphere is Saben silver steel is spherodise desirable. annealed for best machinability, the annealed STOCK SIZES DIA Heat to 770 / 780ºC and when 2 mm 15 1/16” hardness being in the region of thoroughly soaked through, 2.5 16 3/32” 270 Brinell (Rockwell C27). quench in water. (sizes up to 8 3 17 1/8” On hardening and tempering a mm diameter may be oil 3.5 18 5/32” hardness of up to Rockwell hardened from 800 / 810ºC) C64 can be obtained. -
Stress Corrosion Cracking of Welded Joints in High Strength Steels
Stress Corrosion Cracking of Welded Joints in High Strength Steels Variables affecting stress corrosion cracking are studied and conditions recommended for welding and postweld heat treat to obtain maximum resistance to cracking BY T. G. GOOCH ABSTRACT. High strength steels may linear elastic fracture mechanics prin detrimental effect on weld metal SCC suffer a form of stress corrosion ciples using precracked specimens. resistance, although segregation may cracking (SCC) due to hydrogen em Testing was carried out in 3% sodium be particularly significant in precipita brittlement, the hydrogen being liber chloride solution as representative tion hardening systems. SCC failure ated by a cathodic corrosion reaction. of the media causing SCC of high may take place intergranularly, by Most service media will be expected strength steels. Welds were prepared cleavage, or by microvoid coales to liberate hydrogen, and the problem in the experimental alloys and the cence, intergranular failure being affords a considerable drawback to pre-existing crack located in various largely associated with the presence the widespread use of high strength regions of the joint, while samples of twinned martensite and high sus steels. For a number of reasons, fail were also prepared using The Weld ceptibility. The results suggest that ure may be particularly likely when ing Institute weld thermal simulator highest SCC resistance will be ob welding is used for fabrication. Un to reproduce specific heat-affected tained from low carbon, low alloy less the -
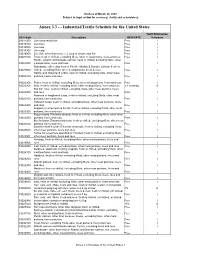
US Schedule for Internet V2
Draft as of March 23, 2007 Subject to legal review for accuracy, clarity and consistency. Annex 3.3 - - Industrial/Textile Schedule for the United States Tariff Elimination US 8 digit Description MFN RATE Schedule 03011000 Live ornamental fish Free I 03019100 Live trout Free I 03019200 Live eels Free I 03019300 Live carp Free I 03019900 Live fish, other than trout, eel, carp or ornamental fish Free I 03021100 Trout, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I Pacific, Atlantic and Danube salmon, fresh or chilled, excluding fillets, other 03021200 meat portions, livers and roes Free I Salmonidae other than trout or Pacific, Atlantic & Danube salmon, fresh or 03021900 chilled, excluding fillets, other meat portions, livers & roes Free I Halibut and Greenland turbot, fresh or chilled, excluding fillets, other meat 03022100 portions, livers and roes Free I 03022200 Plaice, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I 03022300 Sole, fresh or chilled, excluding fillets, other meat portions, livers and roes 1.1 cents/kg A Flat fish, nesi, fresh or chilled, excluding fillets, other meat portions, livers 03022900 and roes Free I Albacore or longfinned tunas, fresh or chilled, excluding fillets, other meat 03023100 portions, livers and roes Free I Yellowfin tunas, fresh or chilled, excluding fillets, other meat portions, livers 03023200 and roes Free I Skipjack or stripe-bellied bonito, fresh or chilled, excluding fillets, other meat 03023300 portions, livers and roes Free -

Carbon and Alloy Steels - Glossary of Terms for Steels
Carbon and Alloy Steels - Glossary of Terms for Steels A Glossary of the terms used in relation to steels and the steel industry. CONTACT Address: Wilsons Ltd Nordic House Old Great North Road Huntingdon PE28 5XN Tel: +44 (0)1480 456421 Email: [email protected] Web: www.wilsonsmetals.com REVISION HISTORY Datasheet Updated 18 July 2019 DISCLAIMER This Data is indicative only and as such is not to be relied upon in place of the full specification. In particular, mechanical property requirements vary widely with temper, product and product dimensions. All information is based on our present knowledge and is given in good faith. No liability will be accepted by the Company in respect of any action taken by any third party in reliance thereon. Please note that the 'Datasheet Update' date shown above is no guarantee of accuracy or whether the datasheet is up to date. The information provided in this datasheet has been drawn from various recognised sources, including EN Standards, recognised industry references (printed & online) and manufacturers’ data. No guarantee is given that the information is from the latest issue of those sources or about the accuracy of those sources. Material supplied by the Company may vary significantly from this data, but will conform to all relevant and applicable standards. As the products detailed may be used for a wide variety of purposes and as the Company has no control over their use; the Company specifically excludes all conditions or warranties expressed or implied by statute or otherwise as to dimensions, properties and/or fitness for any particular purpose, whether expressed or implied. -

Study of Phosphorus Behaviour in Levitated Silicon-Iron Droplets
STUDY OF PHOSPHORUS BEHAVIOUR IN LEVITATED SILICON-IRON DROPLETS by Katherine Le A thesis submitted in conformity with the requirements for the degree of Master of Applied Science Department of Materials Science and Engineering University of Toronto © Copyright by Katherine Le 2016 ii Study of Phosphorus Behaviour in Levitated Si-Fe Droplets Katherine Le Master of Applied Science Department of Materials Science and Engineering University of Toronto 2016 Abstract While the treatment of relatively inexpensive ferrosilicon alloys is a potential refining route in order to generate solar grade silicon, phosphorus is one of the more difficult impurities to remove by conventional processing. In this project, electromagnetic levitation was used to investigate the dephosphorization of ferrosilicon alloy droplets exposed to H2-Ar gas mixtures under various experimental conditions including, refining time, temperature (1450°C-1720°C), H2-Ar gas concentrations and flow rate, iron alloying content, and initial phosphorus concentration. Reaction rates increased with higher refining times, temperatures, and H2 gas concentrations. With unknown parameters associated with the kinetics of gas phase reactions, the approach involved comparison of apparent activation energies derived for the chemical reaction and gas diffusion steps of the dephosphorization process. The phosphorus removal rate is thought to be controlled by the interfacial reaction step; further work is required to confirm this conclusion. iii Acknowledgements I would like to express my gratitude and respect to my supervisor, Prof. Alex McLean for the opportunity to work on this research. I am thankful for his guidance, wisdom and encouragement throughout the course of my studies. He is a truly inspiring person, and a great enabler of new learning opportunities. -

Binary and Ternary Transition-Metal Phosphides As Hydrodenitrogenation Catalysts
Research Collection Doctoral Thesis Binary and ternary transition-metal phosphides as hydrodenitrogenation catalysts Author(s): Stinner, Christoph Publication Date: 2001 Permanent Link: https://doi.org/10.3929/ethz-a-004378279 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss. ETH No. 14422 Binary and Ternary Transition-Metal Phosphides as Hydrodenitrogenation Catalysts A dissertation submitted to the Swiss Federal Institute of Technology Zurich for the degree of Doctor of Natural Sciences Presented by Christoph Stinner Dipl.-Chem. University of Bonn born February 27, 1969 in Troisdorf (NRW), Germany Accepted on the recommendation of Prof. Dr. Roel Prins, examiner Prof. Dr. Reinhard Nesper, co-examiner Dr. Thomas Weber, co-examiner Zurich 2001 I Contents Zusammenfassung V Abstract IX 1 Introduction 1 1.1 Motivation 1 1.2 Phosphides 4 1.2.1 General 4 1.2.2 Classification 4 1.2.3 Preparation 5 1.2.4 Properties 12 1.2.5 Applications and Uses 13 1.3 Scope of the Thesis 14 1.4 References 16 2 Characterization Methods 1 2.1 FT Raman Spectroscopy 21 2.2 Thermogravimetric Analysis 24 2.3 Temperature-Programmed Reduction 25 2.4 X-Ray Powder Diffractometry 26 2.5 Nitrogen Adsorption 28 2.6 Solid State Nuclear Magnetic Resonance Spectroscopy 28 2.7 Catalytic Test 33 2.8 References 36 3 Formation, Structure, and HDN Activity of Unsupported Molybdenum Phosphide 37 3.1 Introduction -

Metals in Horology – a Paper by Jim Nicholson
Metals in Horology – a paper by Jim Nicholson Mans early use of metals Early man exploited gold, silver and copper because these can be found ‘native’ or in the metallic state: subsequently their ores, as well as those of tin, were relatively easily reduced to the metallic state at comparatively low temperature. Iron is very occasionally found in the metallic state in the form of meteorites. It is possible that the relative lack of iron meteorites today, compared with the frequency with which they are believed to have fallen to earth, is because they were exploited by early man. It has been reported that the Inuits of North America used such resources at least to the end of the 19th century. It must have appeared magical to early man that the smelting of rock resulted in producing a metal and that an alloy of two soft metals (copper and tin) could result in a hard alloy capable of bearing a cutting edge (bronze). Brass An alloy of copper and zinc. This seems simple, but it must be remembered that zinc, in a metallic state was only available quite late in history. Copper was produced in the Bristol and Swansea areas in large quantities in the 18th & 19th centuries. At one time 50% of the worlds copper production was done here. Extraction was a complex process involving six or seven separate smelting and the slag from each stage was added to the charge two or three stages later on. Most copper ores are sulphides but also they contained arsenic. The brass making process involved filling crucibles with the trimmings or punchings from the making of the sheet copper, plus zinc carbonate or calamine, which was found in the lead mines of Derbyshire and the Yorkshire Dales, and powdered charcoal. -

The Effect of Temperature and Strain-Rate on the Deformation and Fracture of Mild Steel Charpy Specimens
1 The Effect of Temperature and Strain-Rate on the Deformation and Fracture of Mild Steel Charpy Specimens The,,,;.is Submitted in Suppliction for the Degree of Doctor of Thilosophy by SLoicor. Rodney Uilshaw D.Sc. A.12.3.M. (London) 1961. Deprtmeilt of -.ysicalietallurrsy- Imperial College University of London December 1964 2 ABSTRACT High nitrogen mild steel Charpy specimens were deformed at room temperature in three-point bending; the distribution of plastic deformation revealed by Fry's etch was measured at different applied loads for both substantially plane stress and plane strain conditions. Specimens were deformed to fracture at striker velocities of 0.05, 50 and 30,000 cm/min within the temperature range - 196°C to + 100°C. These studies have revealed the existence of ; a) a transition from ductile tearing at the notch root, to internal cleavage, b) at a lower temperature a bimodal distribution of fracture loads, indicating a transition in the mode of cleavage fracture and c) a decrease in the fracture load associated with the onset of twinning. 3 The relationship between these transition temperatures and the strain-rate may be expressed by an Arrhenius eauation with different apparent activation energies, which are not comparable with the activation energies for yielding. From this it is concluded that the effect of temperature and strain-rate on the cleavage stress is not entirely due to their influence on the yield stress. The implication of this when predicting notch impact transitions from tensile data is disc-p.ssed and a method of predicting the existence of a Crack arrest temperature is postulated. -

A Review of Aluminium Phosphide Poisoning and a Flowchart to Treat It Arh Hig Rada Toksikol 2016;67:183-193 183
Hashemi-Domeneh, et al. A review of aluminium phosphide poisoning and a flowchart to treat it Arh Hig Rada Toksikol 2016;67:183-193 183 Review DOI: 10.1515/aiht-2016-67-2784 A review of aluminium phosphide poisoning and a flowchart to treat it Behrooz Hashemi-Domeneh1,2, Nasim Zamani1,2, Hossein Hassanian-Moghaddam1,2, Mitra Rahimi1,2, Shahin Shadnia1,2, Peyman Erfantalab1,2, and Ali Ostadi1,2 Toxicological Research Center, Department of Clinical Toxicology, Loghman-Hakim Hospital, Shahid Beheshti University of Medical Sciences1, Excellence Center of Clinical Toxicology, Iranian Ministry of Health2, Tehran, Iran [Received in February 2016; CrossChecked in February 2016; Accepted in September 2016] The use of pesticides such as aluminium phosphide (AlP) has increased in the recent years and improved the quantity and quality of agricultural products in a number of developing countries. The downside is that AlP causes severe chronic and acute health effects that have reached major proportions in countries such as India, Iran, Bangladesh, and Jordan. Nearly 300,000 people die due to pesticide poisoning in the world every year. Poisoning with AlP accounts for many of these deaths. Unfortunately, at the same time, there is no standard treatment for it. The aim of this article is to give a brief review of AlP poisoning and propose a treatment flowchart based on the knowledge gained so far. For this purpose we reviewed all articles on the management of AlP poisoning published from 2000 till now. Using a modified Delphi design, we have designed a handy flowchart that could be used as a guide for AlP poisoning management of patients in emergency centres. -

Durham E-Theses
Durham E-Theses An investigation of the magnetic properties of high tensile steels Willcock, Simon Nicolas Murray How to cite: Willcock, Simon Nicolas Murray (1985) An investigation of the magnetic properties of high tensile steels, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/7026/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk AN INVESTIGATION OF THE MAGNETIC PROPERTIES OF HIGH TENSILE STEELS SIMON NICOLAS MURRAY WILLCOCK, B.Sc, A.R.C.S., C. Phys., M.Inst.P, The copyright of this thesis rests with the author. No quotation from it should be published without his prior written consent and information derived from it should be acknowledged. Thesis submitted to the University of Durham in Candidature for the Degree of Doctor of Philosophy, September, 1985. To my Family - i - ABSTRACT This thesis describes an investigation of the magnetic properties of high tensile steels typical of those produced for the high pressure gas pipe-line industry. -

The Silver Standard of Steel
When it comes to the number of steel grades available, we can understand why people are astonished and bemused by the options available! It does seem that there is a steel grade for every single application you can think of, but each one exists for a specific purpose and has its own capabilities, strengths and weaknesses! One that always catches the eye of our customers is our Silver Steel Bar if not only because people immediately ask if there’s any silver contained in it! Unfortunately, there isn’t, it’s a historic name from the first half of the 20th Century, as the chromium that was alloyed into this particular steel gave it an extra bright finish, that made it appear like silver. The good news is that Silver Steel is much stronger and more capable than the silver it’s named after! Silver Steel has a high carbon content, which increases the hardenability during working and allows it to withstand wear much better than other grades. Wear resistance is very important in any number of functions, but the chromium that gives the Silver Steel its lustre also increases the strength. The actual make up of Silver Steel includes a number of other materials. As well as carbon and chromium, there’s also manganese. If you need a strong and hard-wearing steel, hardening and tempering achieves a hardness of up to Rockwell C 64. The chromium content means that the material responds very well to treatment that the process is a deep hardening throughout the material. There are many reasons to choose silver steel bar as your steel of choice, not only for its strength, hardness and machineability, but also because you can tell your clients that you’re using ‘silver steel’ and sound very grand indeed – if you don’t tell, we won’t! Powered by TCPDF (www.tcpdf.org). -

Download SCOPE Newsletter
With participation of the European Commission: DG GROW and Joint Research Centre Summary of joint European Commission – ESPP webinar th on P4 (phosphorus) Critical Raw Material, 9 July 2020 This ESPP SCOPE Newsletter summarises discussions at the expert webinar jointly organised by ESPP and the European Commission on 9th July, with participation of Contents the leading companies using phosphorus (P4) or its derivatives in Europe, with additional input from the The EU Critical Raw Materials: “Phosphate Rock” and “Phosphorus”........................................................................... 2 independent industry expert Willem Schipper. The content (Fig. i) Overview numbers: from phosphate rock to P4 / P4-derivatives below has been seen by the participants but does not and applications .................................................................................. 3 represent their position, it represents ESPP’s own How is P4 produced? the phosphorus furnace .............. 4 assessment. Photo: P4 furnace ................................................................................ 4 Schematic: P4 manufacture process (simplified) ................................. 4 This webinar aimed to provide expert input to the European Which chemicals critically depend on P4 / P4 derivatives? . 5 Commission’s MSA (Material System Analysis) of the EU dependency on P4 concerns many industry sectors . 5 specific industrial form of “Phosphorus”, P4, as a Critical (Fig. ii) Production via wet-acid or via P4 / P4 derivatives / ‘thermal’ Raw