Catalog of Standard Reference Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Silver Steel Data Sheet
SSS SABEN SILVER STEEL 1.2210 PRECISION GROUND DOWELL ROD B.S.1407 WERKSTOFF No C Si Mn Cr Mo W V Co 1.20 0.40 0.40 Features and Uses Tempering Applications include Temper according to the Saben Silver Steel is bright screwdrivers, punches, shafts, purpose for which the parts are finished rod produced from hot axles, pinions, pins, die posts, required generally between rolled bar by means of instrument parts, model parts, 150 / 300ºC centreless grinding. taps and drills for mild steel, The high carbon content of this engravers tools, and fine Rockwell C Temperature steel means that it can be cutters. 63/65 as quenched hardened to give considerable 63/65 120ºC wear resistance and the Hardening 64/62 150ºC chromium content adds to the 62/61 200ºC strength and hardenability It is preferable to heat the tools 59/58 250ºC As supplied however, the steel in a controlled atmosphere. If 56/55 300ºC is machinable owing to the this is not possible, pack 54/53 350ºC annealing treatment given to it hardening is recommended. A 50/48 400ºC prior to grinding. reducing atmosphere is Saben silver steel is spherodise desirable. annealed for best machinability, the annealed STOCK SIZES DIA Heat to 770 / 780ºC and when 2 mm 15 1/16” hardness being in the region of thoroughly soaked through, 2.5 16 3/32” 270 Brinell (Rockwell C27). quench in water. (sizes up to 8 3 17 1/8” On hardening and tempering a mm diameter may be oil 3.5 18 5/32” hardness of up to Rockwell hardened from 800 / 810ºC) C64 can be obtained. -
Stress Corrosion Cracking of Welded Joints in High Strength Steels
Stress Corrosion Cracking of Welded Joints in High Strength Steels Variables affecting stress corrosion cracking are studied and conditions recommended for welding and postweld heat treat to obtain maximum resistance to cracking BY T. G. GOOCH ABSTRACT. High strength steels may linear elastic fracture mechanics prin detrimental effect on weld metal SCC suffer a form of stress corrosion ciples using precracked specimens. resistance, although segregation may cracking (SCC) due to hydrogen em Testing was carried out in 3% sodium be particularly significant in precipita brittlement, the hydrogen being liber chloride solution as representative tion hardening systems. SCC failure ated by a cathodic corrosion reaction. of the media causing SCC of high may take place intergranularly, by Most service media will be expected strength steels. Welds were prepared cleavage, or by microvoid coales to liberate hydrogen, and the problem in the experimental alloys and the cence, intergranular failure being affords a considerable drawback to pre-existing crack located in various largely associated with the presence the widespread use of high strength regions of the joint, while samples of twinned martensite and high sus steels. For a number of reasons, fail were also prepared using The Weld ceptibility. The results suggest that ure may be particularly likely when ing Institute weld thermal simulator highest SCC resistance will be ob welding is used for fabrication. Un to reproduce specific heat-affected tained from low carbon, low alloy less the -

Thesis-1959-M936z.Pdf (5.165Mb)
THE ZING SMETIL'ING INDUSTRY IN OKLAHOMA By ROLAND DELOY MOWER ti Bachelor of Science University of Utah Salt Lake City, Utah 1955 Submitted to the Faculty of the Graduate Sohool of the Oklahoma State University in Partial Fulfillment of the Requirements for the Degree or MASTER OF SCIENCE August, 1959 STATE UNIVERSITY , LIBRARY . NOV 18 1959 1 ..'· TEE ZINC SMELTING mDUSTRY m OKLAHOMA Thesis Approved: tfa/r£~if:i:#d 7 Dean of the Graduate School 430809 ii PREFACE // ·one of the most mportant aspects of the tremendous development and growth of American industry is an increased awareness of the basic metals, their products and their uses o Steel, aluminum and copper are pa.rticu= larly well knO'wn because of the publicity they receive and because their presence is easily recognized in a wide range of consumer products. On the other hand zinc~ which ranks fourth among other metals with respect to production, is relatively unknown. Because zinc is generally used in conjunction with other metals its identity is often hidden and the average person, unaware of zinc's wide application and uses in industry, fails to reeog:nize its significan9e ,/ In this study of Oklahoma vs zinc smelting industry I have attempted to acquaint the average Oklahoman with zinc 1 its sources, products and consumers. The zinc industry as a whole is discussed.,- bttt special emphasis is given to that part of the industry located in Oklahoma. Information contained_ in this thesis was obtained from various published materials found in several libraries :i through personal -
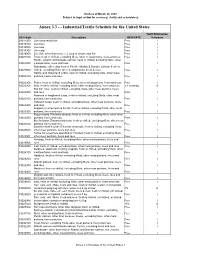
US Schedule for Internet V2
Draft as of March 23, 2007 Subject to legal review for accuracy, clarity and consistency. Annex 3.3 - - Industrial/Textile Schedule for the United States Tariff Elimination US 8 digit Description MFN RATE Schedule 03011000 Live ornamental fish Free I 03019100 Live trout Free I 03019200 Live eels Free I 03019300 Live carp Free I 03019900 Live fish, other than trout, eel, carp or ornamental fish Free I 03021100 Trout, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I Pacific, Atlantic and Danube salmon, fresh or chilled, excluding fillets, other 03021200 meat portions, livers and roes Free I Salmonidae other than trout or Pacific, Atlantic & Danube salmon, fresh or 03021900 chilled, excluding fillets, other meat portions, livers & roes Free I Halibut and Greenland turbot, fresh or chilled, excluding fillets, other meat 03022100 portions, livers and roes Free I 03022200 Plaice, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I 03022300 Sole, fresh or chilled, excluding fillets, other meat portions, livers and roes 1.1 cents/kg A Flat fish, nesi, fresh or chilled, excluding fillets, other meat portions, livers 03022900 and roes Free I Albacore or longfinned tunas, fresh or chilled, excluding fillets, other meat 03023100 portions, livers and roes Free I Yellowfin tunas, fresh or chilled, excluding fillets, other meat portions, livers 03023200 and roes Free I Skipjack or stripe-bellied bonito, fresh or chilled, excluding fillets, other meat 03023300 portions, livers and roes Free -

Carbon and Alloy Steels - Glossary of Terms for Steels
Carbon and Alloy Steels - Glossary of Terms for Steels A Glossary of the terms used in relation to steels and the steel industry. CONTACT Address: Wilsons Ltd Nordic House Old Great North Road Huntingdon PE28 5XN Tel: +44 (0)1480 456421 Email: [email protected] Web: www.wilsonsmetals.com REVISION HISTORY Datasheet Updated 18 July 2019 DISCLAIMER This Data is indicative only and as such is not to be relied upon in place of the full specification. In particular, mechanical property requirements vary widely with temper, product and product dimensions. All information is based on our present knowledge and is given in good faith. No liability will be accepted by the Company in respect of any action taken by any third party in reliance thereon. Please note that the 'Datasheet Update' date shown above is no guarantee of accuracy or whether the datasheet is up to date. The information provided in this datasheet has been drawn from various recognised sources, including EN Standards, recognised industry references (printed & online) and manufacturers’ data. No guarantee is given that the information is from the latest issue of those sources or about the accuracy of those sources. Material supplied by the Company may vary significantly from this data, but will conform to all relevant and applicable standards. As the products detailed may be used for a wide variety of purposes and as the Company has no control over their use; the Company specifically excludes all conditions or warranties expressed or implied by statute or otherwise as to dimensions, properties and/or fitness for any particular purpose, whether expressed or implied. -

The Bristol Brass Industry: Furnace Structures and Their Associated Remains Joan M Day
The Bristol brass industry: Furnace structures and their associated remains Joan M Day Remains of the once-extensive Bristol brass industry failed appear to have been complex. Political and can still be seen at several sites on the banks of the economic developments of the time contributed to A von and its tri butaries between Bath and Bristol.! varying extents. So too, did the availability of raw They are relics of the production of brass and its materials and good sources of fuel and waterpower, but manufacture which nourished during the eighteenth technical innovation in the smelting of copper, which century to become the most important industry of its was being evolved locally, provided a major component kind in Europe, superseding continental centres of of the initial success.3 It laid foundations for Bristol's similar production. By the close of the century Bristol domination of the industry throughout the greater part itself was challenged by strong competition and the of the eighteenth century. adoption of new techniques in Birmingham, and thereafter suffered a slow decline. Still using its Significantly, it was Abraham Oarby who was eighteenth-century water-powered methods the Bristol responsible as 'active man', together with Quaker industry just managed to survive into the twentieth partners, for launching the Bristol company in 1702. century, finally closing in the 1920s.2 After some five years' experience in employing coal• fired techniques in the non-ferrous metals industry he The factors which gave impetus to the growth -

Samuel Wetherill, Joseph Wharton, and the Founding of the ^American Zinc Industry
Samuel Wetherill, Joseph Wharton, and the Founding of the ^American Zinc Industry HE TWO people most closely associated with the founding of the zinc industry in the United States were the Philadel- Tphians Samuel Wetherill (i821-1890) and Joseph Wharton (1826-1909). From 1853 to about i860 they variously cooperated and competed with each other in setting up commercially successful plants for making zinc oxide and metallic zinc for the Pennsylvania and Lehigh Zinc Company at South Bethlehem, Pennsylvania. Both did their work in the face of an established and successful zinc industry in Europe. Accordingly, they looked to Europe for standards governing efficiency of production, quality of product, and the arts of management and marketing. They had to surpass at least some of these standards in order to establish the domestic industry on a firm basis. Zinc is a blue to grey metal found in deposits throughout the world. It is used for thousands of products, for example, in the fields of medicine, cosmetics, die casting alloys, galvanizing of iron, paint, rubber, ceramics, plastics, chemicals, and heavy metals. It ranks "only behind aluminum and copper in order of consumption among the nonferrous metals."1 In short, it has from an early stage in the Industrial Revolution been essential to the maintenance and progress of a technological society. The industry has two main branches. One is the manufacture of zinc oxide. The other is the making of metallic zinc or spelter, as it is called in the trade. These industries are relatively new in the western world. Portuguese and Dutch traders brought spelter to Europe from the Orient about the seventeenth century. -

Study of Phosphorus Behaviour in Levitated Silicon-Iron Droplets
STUDY OF PHOSPHORUS BEHAVIOUR IN LEVITATED SILICON-IRON DROPLETS by Katherine Le A thesis submitted in conformity with the requirements for the degree of Master of Applied Science Department of Materials Science and Engineering University of Toronto © Copyright by Katherine Le 2016 ii Study of Phosphorus Behaviour in Levitated Si-Fe Droplets Katherine Le Master of Applied Science Department of Materials Science and Engineering University of Toronto 2016 Abstract While the treatment of relatively inexpensive ferrosilicon alloys is a potential refining route in order to generate solar grade silicon, phosphorus is one of the more difficult impurities to remove by conventional processing. In this project, electromagnetic levitation was used to investigate the dephosphorization of ferrosilicon alloy droplets exposed to H2-Ar gas mixtures under various experimental conditions including, refining time, temperature (1450°C-1720°C), H2-Ar gas concentrations and flow rate, iron alloying content, and initial phosphorus concentration. Reaction rates increased with higher refining times, temperatures, and H2 gas concentrations. With unknown parameters associated with the kinetics of gas phase reactions, the approach involved comparison of apparent activation energies derived for the chemical reaction and gas diffusion steps of the dephosphorization process. The phosphorus removal rate is thought to be controlled by the interfacial reaction step; further work is required to confirm this conclusion. iii Acknowledgements I would like to express my gratitude and respect to my supervisor, Prof. Alex McLean for the opportunity to work on this research. I am thankful for his guidance, wisdom and encouragement throughout the course of my studies. He is a truly inspiring person, and a great enabler of new learning opportunities. -

Binary and Ternary Transition-Metal Phosphides As Hydrodenitrogenation Catalysts
Research Collection Doctoral Thesis Binary and ternary transition-metal phosphides as hydrodenitrogenation catalysts Author(s): Stinner, Christoph Publication Date: 2001 Permanent Link: https://doi.org/10.3929/ethz-a-004378279 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss. ETH No. 14422 Binary and Ternary Transition-Metal Phosphides as Hydrodenitrogenation Catalysts A dissertation submitted to the Swiss Federal Institute of Technology Zurich for the degree of Doctor of Natural Sciences Presented by Christoph Stinner Dipl.-Chem. University of Bonn born February 27, 1969 in Troisdorf (NRW), Germany Accepted on the recommendation of Prof. Dr. Roel Prins, examiner Prof. Dr. Reinhard Nesper, co-examiner Dr. Thomas Weber, co-examiner Zurich 2001 I Contents Zusammenfassung V Abstract IX 1 Introduction 1 1.1 Motivation 1 1.2 Phosphides 4 1.2.1 General 4 1.2.2 Classification 4 1.2.3 Preparation 5 1.2.4 Properties 12 1.2.5 Applications and Uses 13 1.3 Scope of the Thesis 14 1.4 References 16 2 Characterization Methods 1 2.1 FT Raman Spectroscopy 21 2.2 Thermogravimetric Analysis 24 2.3 Temperature-Programmed Reduction 25 2.4 X-Ray Powder Diffractometry 26 2.5 Nitrogen Adsorption 28 2.6 Solid State Nuclear Magnetic Resonance Spectroscopy 28 2.7 Catalytic Test 33 2.8 References 36 3 Formation, Structure, and HDN Activity of Unsupported Molybdenum Phosphide 37 3.1 Introduction -

II/IV B. Tech (Regular) DEGREE Examinationapril'2018
II/IV B. Tech (Regular) DEGREE EXAMINATIONApril’2018 Mechanical Engineering Casting, Forming and Welding Technology (14 ME 405) Detailed Scheme of Evaluation Max. Marks: 60 -------------------------------------------------------------------------------------------------------------------------------------- 1. Answer all the questions. (2x6=12) (a) Mention any two disadvantages of die casting Answer: 1) All metals and alloys cannot be cast. 2) The cost of machines, dies and other equipment used is high. 3) Not economical for small quantity production. 4) Heavy casting cannot be cast. 5) Special precautions are necessary for evacuation of air from die cavity, otherwise cause porosity. (b) What are the materials that are generally used for preparing patterns? Answer: Patterns may be constructed from the following materials. Each material has its own advantages, limitations, and field of application. Some materials used for making patterns are: wood, metals and alloys, plastic, plaster of Paris, plastic and rubbers, wax, and resins (c) Define the casting yield. Answer: The efficiency, or yield, of a casting is defined as the weight of the casting divided by the weight of the total amount of metal poured (d) What is the most commonly used type of gate? Answer: Horizontal Gating System is used most widely. (e) Specify the advantages of the precision investment casting process. Answer: -Excellent surface finish -High dimensional accuracy -Extremely intricate parts are castable -Almost any metal can be cast -No flash or parting lines (f) How is a semi-permanent mould different from a permanent mould? Answer: In a permanent mold casting there are two molds used and these molds are joined together in which metals are molted when poured in these molds. -

Metals in Horology – a Paper by Jim Nicholson
Metals in Horology – a paper by Jim Nicholson Mans early use of metals Early man exploited gold, silver and copper because these can be found ‘native’ or in the metallic state: subsequently their ores, as well as those of tin, were relatively easily reduced to the metallic state at comparatively low temperature. Iron is very occasionally found in the metallic state in the form of meteorites. It is possible that the relative lack of iron meteorites today, compared with the frequency with which they are believed to have fallen to earth, is because they were exploited by early man. It has been reported that the Inuits of North America used such resources at least to the end of the 19th century. It must have appeared magical to early man that the smelting of rock resulted in producing a metal and that an alloy of two soft metals (copper and tin) could result in a hard alloy capable of bearing a cutting edge (bronze). Brass An alloy of copper and zinc. This seems simple, but it must be remembered that zinc, in a metallic state was only available quite late in history. Copper was produced in the Bristol and Swansea areas in large quantities in the 18th & 19th centuries. At one time 50% of the worlds copper production was done here. Extraction was a complex process involving six or seven separate smelting and the slag from each stage was added to the charge two or three stages later on. Most copper ores are sulphides but also they contained arsenic. The brass making process involved filling crucibles with the trimmings or punchings from the making of the sheet copper, plus zinc carbonate or calamine, which was found in the lead mines of Derbyshire and the Yorkshire Dales, and powdered charcoal. -
![United States Patent [19] [111 3,903,585 Kosteruk Et Al](https://docslib.b-cdn.net/cover/5191/united-states-patent-19-111-3-903-585-kosteruk-et-al-1125191.webp)
United States Patent [19] [111 3,903,585 Kosteruk Et Al
United States Patent [19] [111 3,903,585 Kosteruk et al. [45] Sept. 9, 1975 [54] METHOD OF BRAZING 2.9791313 4/l96l Steinberg ....................... .. 29/504 X [761 ‘""emors: vakmi" pe‘mvkh Kosm'uki “msa 520918063 44 [3119231 19 gdulerifeurreta] e . .al. ........v . e . ...t 23:28: i M- Krlvonosa, 19» kv- 5;_Mikhail 3,442,006 5/l969 Grucket et a1...‘ 29/4711 x Savvich Kovalchenko. ulltsa 3,594,895 7/1971 Hill ....................... .. 29/504 x Kapitanovskaya, l0, kv. 20, both of 3,736,648 6/1973 Spielberg ......................... t. 29/4731 Kiev, U.S.S.R. [22] Filed: Ann 27, 1972 Primary Examiner—Francis S. Husar Assistant Examiner—Ronald J. Shore [2]] APPL N03 248,295 Attorney, Agent, or Firm—Waters, Schwartz & Nissen Related U.S. Application Data [63] Continuation of Ser. No. 875,503, Nov. l0, i969, [57] ABSTRACT abandoned‘ lnfusible metal alloys are employed both as a parts I ‘ material and as a brazing spelter for the connection of [52] U.S. Cl. ...... .... .. 75/134 V, 2222880122623, various parts and componems made of materialsrbascd [5 H I ‘ C‘ ‘ 823k suoz upon infusible metals and compounds, ceramics. [58 F’: ‘Id """" 473 1 graphite and the like. The alloys are based upon haf ] e o arc "" " ' ‘29/4729’ 477i 7’ nium to which is added some elements selected from ‘ ' ' the subgroup B of the ?rst group of the periodic sys tem and some elements featuring melting points lying [56] References Cited above 600°C and selected from the third to eighth UNITED STATES PATENTS groups of the periodic system.