Tariff List – September 17, 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hexamethylenediamine (HMDA) from Fossil Vs. Bio-Based Routes: an Economic and Life Cycle Assessment Comparative Study
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is © The Royal Society of Chemistry 2015 Hexamethylenediamine (HMDA) from Fossil vs. Bio-Based Routes: an Economic and Life Cycle Assessment Comparative Study A. B. Dros,b O. Larue,b A. Reimond,b F. De Campoa and M. Pera-Titusa* a Eco-Efficient Products and Processes Laboratory (E2P2L), UMI 3464 CNRS-Solvay, 3966 Jin Du Road, Xin Zhuang Ind. Zone, 201108 Shanghai, China. b Solvay (China) Co., Ltd., 3966 Jin Du Rd., Xin Zhuang Industrial Zone, Shanghai 201108, PR China. * Corresponding author. Tel.: +86 (0) 472445368, Fax: +86 (0) 472445399, E-mail: marc.pera-titus- [email protected] ELECTRONIC SUPPORTING INFORMATION FIGURE AND TABLE CAPTIONS Fig. S1. Speculative routes for the production of bio-based HMDA using molecules issued from biomass. Fig. S2. Flowsheet for the fossil-based route 1. Fig. S3. Flowsheet for the route Starch HFS. The bold number corresponds to the base-case value for steam consumption used in the LCA sensitivity study. The w/o HFS drying scenario value is also indicated. Fig. S4. Flowsheet for the bio-based route 2. Fig. S5. Flowsheet for the bio-based route 3. The bold numbers correspond to the base-case values used in the LCA sensitivity study. For HFS, the best- and worst case scenario values are also indicated. Fig. S6. Flowsheet for the bio-based route 4. Fig. S7. Evolution of market price of butadiene and HFCS42%. Data obtained from ref.18 and ref.21, respectively. Fig. S8. Impact score breakdown for ozone depletion (midpoint category 2) for HMDA production in France and Germany. -

Aldrich FT-IR Collection Edition I Library
Aldrich FT-IR Collection Edition I Library Library Listing – 10,505 spectra This library is the original FT-IR spectral collection from Aldrich. It includes a wide variety of pure chemical compounds found in the Aldrich Handbook of Fine Chemicals. The Aldrich Collection of FT-IR Spectra Edition I library contains spectra of 10,505 pure compounds and is a subset of the Aldrich Collection of FT-IR Spectra Edition II library. All spectra were acquired by Sigma-Aldrich Co. and were processed by Thermo Fisher Scientific. Eight smaller Aldrich Material Specific Sub-Libraries are also available. Aldrich FT-IR Collection Edition I Index Compound Name Index Compound Name 3515 ((1R)-(ENDO,ANTI))-(+)-3- 928 (+)-LIMONENE OXIDE, 97%, BROMOCAMPHOR-8- SULFONIC MIXTURE OF CIS AND TRANS ACID, AMMONIUM SALT 209 (+)-LONGIFOLENE, 98+% 1708 ((1R)-ENDO)-(+)-3- 2283 (+)-MURAMIC ACID HYDRATE, BROMOCAMPHOR, 98% 98% 3516 ((1S)-(ENDO,ANTI))-(-)-3- 2966 (+)-N,N'- BROMOCAMPHOR-8- SULFONIC DIALLYLTARTARDIAMIDE, 99+% ACID, AMMONIUM SALT 2976 (+)-N-ACETYLMURAMIC ACID, 644 ((1S)-ENDO)-(-)-BORNEOL, 99% 97% 9587 (+)-11ALPHA-HYDROXY-17ALPHA- 965 (+)-NOE-LACTOL DIMER, 99+% METHYLTESTOSTERONE 5127 (+)-P-BROMOTETRAMISOLE 9590 (+)-11ALPHA- OXALATE, 99% HYDROXYPROGESTERONE, 95% 661 (+)-P-MENTH-1-EN-9-OL, 97%, 9588 (+)-17-METHYLTESTOSTERONE, MIXTURE OF ISOMERS 99% 730 (+)-PERSEITOL 8681 (+)-2'-DEOXYURIDINE, 99+% 7913 (+)-PILOCARPINE 7591 (+)-2,3-O-ISOPROPYLIDENE-2,3- HYDROCHLORIDE, 99% DIHYDROXY- 1,4- 5844 (+)-RUTIN HYDRATE, 95% BIS(DIPHENYLPHOSPHINO)BUT 9571 (+)-STIGMASTANOL -

Hexamethylenediamine Cas N°: 124-09-4
OECD SIDS HEXAMETHYLENEDIAMINE FOREWORD INTRODUCTION HEXAMETHYLENEDIAMINE CAS N°: 124-09-4 UNEP PUBLICATIONS Identifiers, Physical and Chemical properties 165 Substance End Point : IDENTIFIERS, PHYSICAL AND CHEMICAL PROPERTIES Chemical Name : 1,6-Hexanediamine Common Name : Hexamethylenediamine CAS Number : 124-09-4 RTECS Number : MO1180000 Synonyms 1,6-Diaminohexane .alpha.-.omega.-Hexanediamine Hexylenediamine 1,6-Hexylenediamine HMD HMDA Properties & Definitions Molecular Formula : C6H16N2 Molecular Weight : 116.24 Melting Point : 41C Boiling Point : 205C Flash Point : 0.9 - 7.6 volume % Vapour Pressure : 0.05 kPa (0.4 mmHg) at 25C CAL Octanol/Water Partition : log Pow = 0.02 Coefficient Water Solubility : 800 g/L at 15.6C Additives : None Impurities : None. Purity of industrial product: 100% General Comments : Flammability (solids/gases): 85C. Ignition temperature: 305C. Overall Evaluation SIDS INITIAL ASSESSMENT CURRENTLY OF LOW PRIORITY FOR FURTHER WORK Hexamethylendiamine (HMDA) is an isolated chemical intermediate which is used for the manufacture of polyamides. Information regarding uses, production levels, exposure, and emissions was available only from the DuPont Company in Canada and in the United States. Tasks involving the exposure to HMDA are of short duration; therefore, occupational exposure is expected to be limited. Air monitoring at plant sites has detected =< 0.07 ppm HMDA; personal monitoring values range from 0.01 to 3.7 ppm. It is also expected that consumer exposure is negligible since HMDA is generally incorporated into other products before reaching the consumer; however, consumer exposure will need to be reassessed when additional exposure data is received from other countries. Under environmental conditions, HMDA will exist in an ionic state (+2). -
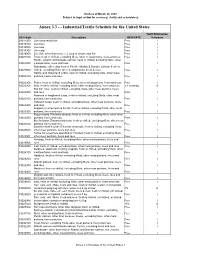
US Schedule for Internet V2
Draft as of March 23, 2007 Subject to legal review for accuracy, clarity and consistency. Annex 3.3 - - Industrial/Textile Schedule for the United States Tariff Elimination US 8 digit Description MFN RATE Schedule 03011000 Live ornamental fish Free I 03019100 Live trout Free I 03019200 Live eels Free I 03019300 Live carp Free I 03019900 Live fish, other than trout, eel, carp or ornamental fish Free I 03021100 Trout, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I Pacific, Atlantic and Danube salmon, fresh or chilled, excluding fillets, other 03021200 meat portions, livers and roes Free I Salmonidae other than trout or Pacific, Atlantic & Danube salmon, fresh or 03021900 chilled, excluding fillets, other meat portions, livers & roes Free I Halibut and Greenland turbot, fresh or chilled, excluding fillets, other meat 03022100 portions, livers and roes Free I 03022200 Plaice, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I 03022300 Sole, fresh or chilled, excluding fillets, other meat portions, livers and roes 1.1 cents/kg A Flat fish, nesi, fresh or chilled, excluding fillets, other meat portions, livers 03022900 and roes Free I Albacore or longfinned tunas, fresh or chilled, excluding fillets, other meat 03023100 portions, livers and roes Free I Yellowfin tunas, fresh or chilled, excluding fillets, other meat portions, livers 03023200 and roes Free I Skipjack or stripe-bellied bonito, fresh or chilled, excluding fillets, other meat 03023300 portions, livers and roes Free -

Chemical Trends in Solid Alkali Pertechnetates † ‡ ‡ ‡ ‡ ‡ Jamie Weaver, , Chuck Z
This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes. Article pubs.acs.org/IC Chemical Trends in Solid Alkali Pertechnetates † ‡ ‡ ‡ ‡ ‡ Jamie Weaver, , Chuck Z. Soderquist, Nancy M. Washton, Andrew S. Lipton, Paul L. Gassman, § ∥ † † ‡ ⊥ Wayne W. Lukens, Albert A. Kruger, Nathalie A. Wall, and John S. McCloy*, , , † Department of Chemistry, Washington State University, Pullman, Washington 99164, United States ‡ Pacific Northwest National Laboratory, Richland, Washington 99352, United States § Lawrence Berkeley National Laboratory, Berkeley, California 94720, United States ∥ U.S. Department of Energy (DOE), Office of River Protection, Richland, Washington 99352, United States ⊥ Materials Science and Engineering Program and School of Mechanical & Materials Engineering, Washington State University, Pullman, Washington 99164, United States *S Supporting Information ABSTRACT: Insight into the solid-state chemistry of pure technetium-99 (99Tc) oxides is required in the development of a robust immobilization and disposal system for nuclear waste stemming from the radiopharmaceutical industry, from the production of nuclear weapons, and from spent nuclear fuel. However, because of its radiotoxicity and the subsequent requirement of special facilities and handling procedures for research, only a few studies have been completed, many of which are over 20 years old. In this study, we report the synthesis of pure alkali pertechnetates (sodium, potassium, rubidium, and cesium) and analysis of these compounds by Raman spectroscopy, X-ray absorption spectroscopy (XANES and EXAFS), solid-state nuclear magnetic resonance (static and magic angle spinning), and neutron diffraction. The structures and spectral signatures of these compounds will aid in refining the understanding of 99Tc incorporation into and release from nuclear waste glasses. -

United States Patent Office Patented Feb
3,235,600 United States Patent Office Patented Feb. 15, 1966 2 quality and color stability of the polyamide product re 3,235,600 Sulting from the polycondensation of hexamethylenedi REDUCTION OF DAMENOCYCLOHEXANE CON. CENTRATION IN CRUDE HEXAMETYLENED amine with dibasic carboxylic acids. The 1,2-diamino AMNE cyclohexane gives rise to salt solutions of hexamethylene Philip W. Evans, Pensacola, Fla., assignor to Monsanto diamine and dibasic carboxylic acids which have poor Company, a corporation of Delaware and variable color, and the polyamides prepared from No Drawing. Filed Nov. 28, 1962, Ser. No. 240,742 these Salt solutions not only have poor color but also are characterized by irregular tensile strength and nonuniform - 4 Claims. (C. 260-583) dyeing properties. This invention relates to the production of amines and Although one major cause for polyamide product more particularly, it relates to a process for the produc 10 quality, color, and dyeing problems has been ascertained tion of amines in a high degree of purity. and explained, the saisfactory removal on a commercial Hexamethylenediamine is now a well-known com Scale of the Small amounts of 1,2-diaminocyclohexane pound which may be prepared on a commercial scale present in manufactured crude hexamethylenediamine to most conveniently by catalytically hydrogenating adipo eliminate the cause presents a difficult problem. Accord nitrile in the presence of ammonia. A principal use of ing to present commercial practices, the 1,2-diaminocyclo hexamethylenediamine involves condensing -

Study of Phosphorus Behaviour in Levitated Silicon-Iron Droplets
STUDY OF PHOSPHORUS BEHAVIOUR IN LEVITATED SILICON-IRON DROPLETS by Katherine Le A thesis submitted in conformity with the requirements for the degree of Master of Applied Science Department of Materials Science and Engineering University of Toronto © Copyright by Katherine Le 2016 ii Study of Phosphorus Behaviour in Levitated Si-Fe Droplets Katherine Le Master of Applied Science Department of Materials Science and Engineering University of Toronto 2016 Abstract While the treatment of relatively inexpensive ferrosilicon alloys is a potential refining route in order to generate solar grade silicon, phosphorus is one of the more difficult impurities to remove by conventional processing. In this project, electromagnetic levitation was used to investigate the dephosphorization of ferrosilicon alloy droplets exposed to H2-Ar gas mixtures under various experimental conditions including, refining time, temperature (1450°C-1720°C), H2-Ar gas concentrations and flow rate, iron alloying content, and initial phosphorus concentration. Reaction rates increased with higher refining times, temperatures, and H2 gas concentrations. With unknown parameters associated with the kinetics of gas phase reactions, the approach involved comparison of apparent activation energies derived for the chemical reaction and gas diffusion steps of the dephosphorization process. The phosphorus removal rate is thought to be controlled by the interfacial reaction step; further work is required to confirm this conclusion. iii Acknowledgements I would like to express my gratitude and respect to my supervisor, Prof. Alex McLean for the opportunity to work on this research. I am thankful for his guidance, wisdom and encouragement throughout the course of my studies. He is a truly inspiring person, and a great enabler of new learning opportunities. -

Binary and Ternary Transition-Metal Phosphides As Hydrodenitrogenation Catalysts
Research Collection Doctoral Thesis Binary and ternary transition-metal phosphides as hydrodenitrogenation catalysts Author(s): Stinner, Christoph Publication Date: 2001 Permanent Link: https://doi.org/10.3929/ethz-a-004378279 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss. ETH No. 14422 Binary and Ternary Transition-Metal Phosphides as Hydrodenitrogenation Catalysts A dissertation submitted to the Swiss Federal Institute of Technology Zurich for the degree of Doctor of Natural Sciences Presented by Christoph Stinner Dipl.-Chem. University of Bonn born February 27, 1969 in Troisdorf (NRW), Germany Accepted on the recommendation of Prof. Dr. Roel Prins, examiner Prof. Dr. Reinhard Nesper, co-examiner Dr. Thomas Weber, co-examiner Zurich 2001 I Contents Zusammenfassung V Abstract IX 1 Introduction 1 1.1 Motivation 1 1.2 Phosphides 4 1.2.1 General 4 1.2.2 Classification 4 1.2.3 Preparation 5 1.2.4 Properties 12 1.2.5 Applications and Uses 13 1.3 Scope of the Thesis 14 1.4 References 16 2 Characterization Methods 1 2.1 FT Raman Spectroscopy 21 2.2 Thermogravimetric Analysis 24 2.3 Temperature-Programmed Reduction 25 2.4 X-Ray Powder Diffractometry 26 2.5 Nitrogen Adsorption 28 2.6 Solid State Nuclear Magnetic Resonance Spectroscopy 28 2.7 Catalytic Test 33 2.8 References 36 3 Formation, Structure, and HDN Activity of Unsupported Molybdenum Phosphide 37 3.1 Introduction -

Red-Light Leds for Next-Generation Displays 6 July 2020
Red-light LEDs for next-generation displays 6 July 2020 indium gallium nitride (InGaN) semiconductors. Integrating two material systems is difficult. "Creating RGB displays requires the mass transfer of the separate blue, green and red LEDs together," says KAUST researcher Zhe Zhuang. An easier solution would be to create different-colored LEDs all on a single semiconductor chip. Optimizing the geometry, fabrication and electrical contacts is vital to maximizing the efficiency of the LED. Credit: Zhuag et al. Novel red LEDs are more temperature stable than those made using the conventional semiconductor of choice. In efforts to optimize the performance of light- emitting diodes (LEDs), King Abdullah University of Science and Technology researchers are looking at every aspect of the design, fabrication and operation of these devices. Now, they have succeeded in fabricating red LEDs, based on the The team developed an InGaN red LED structure where naturally blue-emitting semiconductor indium the output power is more stable than that of InGaP red gallium nitride, that are as stable as those based LEDs. Credit: Zhe Zhuang on indium gallium phosphide. LEDs are optical sources made from semiconductors that offer improvements on Since InGaP semiconductors are unable to emit conventional visible-light sources in terms of blue or green light, the only solution to making energy saving, smaller size and longer lifetimes. monolithic RGB micro-LEDs is to use InGaN. This LEDs can emit across the spectrum, from the material has the potential to shift its emission from ultraviolet to blue (B), green (G), red (R) and into blue to green, yellow and red by introducing more the infrared. -

Harmonized Tariff Schedule of the United States (2020) Revision 14 Annotated for Statistical Reporting Purposes
Harmonized Tariff Schedule of the United States (2020) Revision 14 Annotated for Statistical Reporting Purposes SECTION VI PRODUCTS OF THE CHEMICAL OR ALLIED INDUSTRIES VI-1 Notes 1. (a) Goods (other than radioactive ores) answering to a description in heading 2844 or 2845 are to be classified in those headings and in no other heading of the tariff schedule. (b) Subject to paragraph (a) above, goods answering to a description in heading 2843, 2846 or 2852 are to be classified in those headings and in no other heading of this section. 2. Subject to note 1 above, goods classifiable in heading 3004, 3005, 3006, 3212, 3303, 3304, 3305, 3306, 3307, 3506, 3707 or 3808 by reason of being put up in measured doses or for retail sale are to be classified in those headings and in no other heading of the tariff schedule. 3. Goods put up in sets consisting of two or more separate constituents, some or all of which fall in this section and are intended to be mixed together to obtain a product of section VI or VII, are to be classified in the heading appropriate to that product, provided that the constituents are: (a) Having regard to the manner in which they are put up, clearly identifiable as being intended to be used together without first being repacked; (b) Entered together; and (c) Identifiable, whether by their nature or by the relative proportions in which they are present, as being complementary one to another. Additional U.S. Notes 1. In determining the amount of duty applicable to a solution of a single compound in water subject to duty in this section at a specific rate, an allowance in weight or volume, as the case may be, shall be made for the water in excess of any water of crystallization which may be present in the undissolved compound. -

P'aturan Harga Satuan Standar BATAN-2009
BADAN TENAGA NUKLIR NASIONAL PERATURAN KEPALA BADAN TENAGA NUKLIR NASIONAL NOMOR: 110/KA/VI/2008 VIII/2007 TENTANG HARGA SATUAN STANDAR BATAN TAHUN ANGGARAN 2009 DENGAN RAHMAT TUHAN YANG MAHA ESA KEPALA BADAN TENAGA NUKLIR NASIONAL, Menimbang : a. bahwa dengan Peraturan Kepala BATAN Nomor 122/KA/VIII/2007 telah ditetapkan Harga Satuan Standar BATAN Tahun Anggaran 2008; b. bahwa mengingat banyaknya perubahan jumlah maupun struktur biaya yang terjadi, akan berdampak terhadap pelaksanaan kegiatan Tahun Anggaran 2009; c. bahwa berdasarkan pertimbangan sebagaimana dimaksud dalam huruf a dan huruf b, perlu menetapkan Peraturan Kepala BATAN tentang Harga Satuan Standar BATAN Tahun Anggaran 2009; Mengingat : 1. Undang-Undang Nomor 17 Tahun 2003 tentang Keuangan Negara (Lembaran Negara Republik Indonesia Tahun 2003 Nomor 47, Tambahan Lembaran Negara Republik Indonesia Nomor 4286); 2. Undang-Undang Nomor 1 Tahun 2004 tentang Perbendaharaan Negara (Lembaran Negara Republik Indonesia Tahun 2004 Nomor 5, Tambahan Lembaran Negara Republik Indonesia Nomor 4355); 3. Undang-Undang Nomor 25 Tahun 2004 tentang Sistem Perencanaan Pembangunan Nasional (Lembaran Negara Republik Indonesia Tahun 2004 Nomor 104, Tambahan Lembaran Negara Republik Indonesia Nomor 4421); 4. Peraturan Pemerintah Nomor 21 Tahun 2004 tentang Penyusunan Rencana Kerja dan Anggaran Kementerian Negara/Lembaga (Lembaran Negara Republik Indonesia Tahun 2004 Nomor 75, Tambahan Lembaran Negara Republik Indonesia Nomor 4406); BADAN TENAGA NUKLIR NASIONAL -2- 5. Peraturan Pemerintah Nomor 77 Tahun 2005 tentang Jenis dan Tarif Atas Jenis Penerimaan Negara Bukan Pajak Yang Berlaku Pada Badan Tenaga Nuklir Nasional (Lembaran Negara Republik Indonesia Tahun 2005 Nomor 163, Tambahan Lembaran Negara Republik Indonesia Nomor 4591); 6. Keputusan Presiden Nomor 103 Tahun 2001 tentang Kedudukan, Tugas, Fungsi, Kewenangan, Susunan Organisasi, dan Tata Kerja Lembaga Pemerintah Non Departemen sebagaimana telah beberapa kali diubah, terakhir dengan Peraturan Presiden Nomor 64 Tahun 2005; 7. -

Reducing Bow of Ingap on Silicon Wafers
94 Technology focus: III-Vs on silicon Reducing bow of InGaP on silicon wafers Researchers use strain engineering without impacting dislocation density. esearchers based in Singapore and the USA have been working to control the wafer bow of Rindium gallium phosphide (InGaP) epitaxial lay- ers on 200mm silicon (Si) wafers [Bing Wang et al, Semicond. Sci. Technol., vol32, p125013, 2017]. “Based on these Wafer bow is caused by observations, we can stress arising mainly conclude that the from mismatches of threading dislocation coefficients of thermal expansion between densities of the InGaP InGaP, or other III-V wafers are not compound semiconduc- affected by the lattice tors, and silicon. The mismatch. Our Ge bow (more than 200µm buffers have similar in one recent report of gallium arsenide on threading dislocation 300mm silicon wafer) is density of 3x107/cm2. introduced when the The hetero-epitaxy of material cools after high- GaAs buffers and temperature epitaxial Figure 1. Epitaxial layer structure of three InGaP/Si deposition. Bowing InGaP films did not wafers. adversely affects wafer- increase the scale processing, partic- threading dislocation was by metal-organic chemical vapor deposition ularly for large-diameter density, which (MOCVD). The germanium on silicon template layer substrates. Wafer-scale was prepared separately in a two-step low/high-tem- indicates very good equipment typically perature process, using germane (GeH4) precursor. restricts the permitted epitaxy quality.” Plan-view transmission electron microscopy (PV-TEM) bow to less than 50µm. The team believes and etch pit density (EPD) analysis gave an estimate of 7 2 The team from the that the technique dislocation density of the order 3x10 /cm .