United States Patent Office Patented July 2, 1963
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Transition Metal Phosphides for the Catalytic Hydrodeoxygenation of Waste Oils Into Green Diesel
catalysts Review Transition Metal Phosphides for the Catalytic Hydrodeoxygenation of Waste Oils into Green Diesel M. Consuelo Alvarez-Galvan * , Jose M. Campos-Martin * and Jose L. G. Fierro * Energy and Sustainable Chemistry Group (EQS), Instituto de Catálisis y Petroleoquímica, CSIC, c/Marie Curie, 2 Cantoblanco, 28049 Madrid, Spain * Correspondence: [email protected] (M.C.A.-G.); [email protected] (J.M.C.-M.); jlgfi[email protected] (J.L.G.F.) Received: 28 February 2019; Accepted: 15 March 2019; Published: 22 March 2019 Abstract: Recently, catalysts based on transition metal phosphides (TMPs) have attracted increasing interest for their use in hydrodeoxygenation (HDO) processes destined to synthesize biofuels (green or renewable diesel) from waste vegetable oils and fats (known as hydrotreated vegetable oils (HVO)), or from bio-oils. This fossil-free diesel product is produced completely from renewable raw materials with exceptional quality. These efficient HDO catalysts present electronic properties similar to noble metals, are cost-efficient, and are more stable and resistant to the presence of water than other classical catalytic formulations used for hydrotreatment reactions based on transition metal sulfides, but they do not require the continuous supply of a sulfide source. TMPs develop a bifunctional character (metallic and acidic) and present tunable catalytic properties related to the metal type, phosphorous-metal ratio, support nature, texture properties, and so on. Here, the recent progress in TMP-based catalysts for HDO of waste oils is reviewed. First, the use of TMPs in catalysis is addressed; then, the general aspects of green diesel (from bio-oils or from waste vegetable oils and fats) production by HDO of nonedible oil compounds are presented; and, finally, we attempt to describe the main advances in the development of catalysts based on TMPs for HDO, with an emphasis on the influence of the nature of active phases and effects of phosphorous, promoters, and preparation methods on reactivity. -
US Schedule for Internet V2
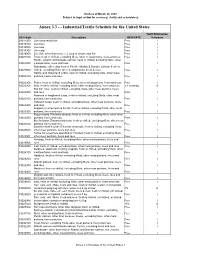
Draft as of March 23, 2007 Subject to legal review for accuracy, clarity and consistency. Annex 3.3 - - Industrial/Textile Schedule for the United States Tariff Elimination US 8 digit Description MFN RATE Schedule 03011000 Live ornamental fish Free I 03019100 Live trout Free I 03019200 Live eels Free I 03019300 Live carp Free I 03019900 Live fish, other than trout, eel, carp or ornamental fish Free I 03021100 Trout, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I Pacific, Atlantic and Danube salmon, fresh or chilled, excluding fillets, other 03021200 meat portions, livers and roes Free I Salmonidae other than trout or Pacific, Atlantic & Danube salmon, fresh or 03021900 chilled, excluding fillets, other meat portions, livers & roes Free I Halibut and Greenland turbot, fresh or chilled, excluding fillets, other meat 03022100 portions, livers and roes Free I 03022200 Plaice, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I 03022300 Sole, fresh or chilled, excluding fillets, other meat portions, livers and roes 1.1 cents/kg A Flat fish, nesi, fresh or chilled, excluding fillets, other meat portions, livers 03022900 and roes Free I Albacore or longfinned tunas, fresh or chilled, excluding fillets, other meat 03023100 portions, livers and roes Free I Yellowfin tunas, fresh or chilled, excluding fillets, other meat portions, livers 03023200 and roes Free I Skipjack or stripe-bellied bonito, fresh or chilled, excluding fillets, other meat 03023300 portions, livers and roes Free -

Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life
Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life Item Type text; Electronic Dissertation Authors Pasek, Matthew Adam Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 07/10/2021 06:16:37 Link to Item http://hdl.handle.net/10150/194288 PHOSPHORUS AND SULFUR COSMOCHEMISTRY: IMPLICATIONS FOR THE ORIGINS OF LIFE by Matthew Adam Pasek ________________________ A Dissertation Submitted to the Faculty of the DEPARTMENT OF PLANETARY SCIENCE In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College UNIVERSITY OF ARIZONA 2 0 0 6 2 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Matthew Adam Pasek entitled Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy _______________________________________________________________________ Date: 04/11/2006 Dante Lauretta _______________________________________________________________________ Date: 04/11/2006 Timothy Swindle _______________________________________________________________________ Date: 04/11/2006 -

Study of Phosphorus Behaviour in Levitated Silicon-Iron Droplets
STUDY OF PHOSPHORUS BEHAVIOUR IN LEVITATED SILICON-IRON DROPLETS by Katherine Le A thesis submitted in conformity with the requirements for the degree of Master of Applied Science Department of Materials Science and Engineering University of Toronto © Copyright by Katherine Le 2016 ii Study of Phosphorus Behaviour in Levitated Si-Fe Droplets Katherine Le Master of Applied Science Department of Materials Science and Engineering University of Toronto 2016 Abstract While the treatment of relatively inexpensive ferrosilicon alloys is a potential refining route in order to generate solar grade silicon, phosphorus is one of the more difficult impurities to remove by conventional processing. In this project, electromagnetic levitation was used to investigate the dephosphorization of ferrosilicon alloy droplets exposed to H2-Ar gas mixtures under various experimental conditions including, refining time, temperature (1450°C-1720°C), H2-Ar gas concentrations and flow rate, iron alloying content, and initial phosphorus concentration. Reaction rates increased with higher refining times, temperatures, and H2 gas concentrations. With unknown parameters associated with the kinetics of gas phase reactions, the approach involved comparison of apparent activation energies derived for the chemical reaction and gas diffusion steps of the dephosphorization process. The phosphorus removal rate is thought to be controlled by the interfacial reaction step; further work is required to confirm this conclusion. iii Acknowledgements I would like to express my gratitude and respect to my supervisor, Prof. Alex McLean for the opportunity to work on this research. I am thankful for his guidance, wisdom and encouragement throughout the course of my studies. He is a truly inspiring person, and a great enabler of new learning opportunities. -

Binary and Ternary Transition-Metal Phosphides As Hydrodenitrogenation Catalysts
Research Collection Doctoral Thesis Binary and ternary transition-metal phosphides as hydrodenitrogenation catalysts Author(s): Stinner, Christoph Publication Date: 2001 Permanent Link: https://doi.org/10.3929/ethz-a-004378279 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss. ETH No. 14422 Binary and Ternary Transition-Metal Phosphides as Hydrodenitrogenation Catalysts A dissertation submitted to the Swiss Federal Institute of Technology Zurich for the degree of Doctor of Natural Sciences Presented by Christoph Stinner Dipl.-Chem. University of Bonn born February 27, 1969 in Troisdorf (NRW), Germany Accepted on the recommendation of Prof. Dr. Roel Prins, examiner Prof. Dr. Reinhard Nesper, co-examiner Dr. Thomas Weber, co-examiner Zurich 2001 I Contents Zusammenfassung V Abstract IX 1 Introduction 1 1.1 Motivation 1 1.2 Phosphides 4 1.2.1 General 4 1.2.2 Classification 4 1.2.3 Preparation 5 1.2.4 Properties 12 1.2.5 Applications and Uses 13 1.3 Scope of the Thesis 14 1.4 References 16 2 Characterization Methods 1 2.1 FT Raman Spectroscopy 21 2.2 Thermogravimetric Analysis 24 2.3 Temperature-Programmed Reduction 25 2.4 X-Ray Powder Diffractometry 26 2.5 Nitrogen Adsorption 28 2.6 Solid State Nuclear Magnetic Resonance Spectroscopy 28 2.7 Catalytic Test 33 2.8 References 36 3 Formation, Structure, and HDN Activity of Unsupported Molybdenum Phosphide 37 3.1 Introduction -

Chemical Vapor Deposition of Heteroepitaxial Boron Phosphide Thin Films
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2013 Chemical Vapor Deposition of Heteroepitaxial Boron Phosphide Thin Films John Daniel Brasfield University of Tennessee - Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Semiconductor and Optical Materials Commons Recommended Citation Brasfield, John Daniel, "Chemical aporV Deposition of Heteroepitaxial Boron Phosphide Thin Films. " PhD diss., University of Tennessee, 2013. https://trace.tennessee.edu/utk_graddiss/2558 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by John Daniel Brasfield entitled "Chemical Vapor Deposition of Heteroepitaxial Boron Phosphide Thin Films." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Chemistry. Charles S. Feigerle, Major Professor We have read this dissertation and recommend its acceptance: Laurence Miller, Frank Vogt, Ziling Xue Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Chemical Vapor Deposition of Heteroepitaxial Boron Phosphide Thin Films A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville John Daniel Brasfield December 2013 Copyright © 2013 by John Daniel Brasfield All rights reserved. -

Download SCOPE Newsletter
With participation of the European Commission: DG GROW and Joint Research Centre Summary of joint European Commission – ESPP webinar th on P4 (phosphorus) Critical Raw Material, 9 July 2020 This ESPP SCOPE Newsletter summarises discussions at the expert webinar jointly organised by ESPP and the European Commission on 9th July, with participation of Contents the leading companies using phosphorus (P4) or its derivatives in Europe, with additional input from the The EU Critical Raw Materials: “Phosphate Rock” and “Phosphorus”........................................................................... 2 independent industry expert Willem Schipper. The content (Fig. i) Overview numbers: from phosphate rock to P4 / P4-derivatives below has been seen by the participants but does not and applications .................................................................................. 3 represent their position, it represents ESPP’s own How is P4 produced? the phosphorus furnace .............. 4 assessment. Photo: P4 furnace ................................................................................ 4 Schematic: P4 manufacture process (simplified) ................................. 4 This webinar aimed to provide expert input to the European Which chemicals critically depend on P4 / P4 derivatives? . 5 Commission’s MSA (Material System Analysis) of the EU dependency on P4 concerns many industry sectors . 5 specific industrial form of “Phosphorus”, P4, as a Critical (Fig. ii) Production via wet-acid or via P4 / P4 derivatives / ‘thermal’ Raw -

Catalog of Standard Reference Materials
NATL INST. OF STAND & TECH Bureau of Standards £-01 Admin. Bldg. AUG 1 8 1970 NBS SPECIAL PUBLICATION 260 NBS JULY 1970 EDITION PUBLICATIONS Catalog of 9TAMDARD "REFERENCE MATERIALS U.S. DEPARTMENT OF COMMERCE National Bureau of Standards - NATIONAL BUREAU OF STANDARDS The National Bureau of Standards 1 was established by an act of Congress March 3, 1901. Today, in addition to serving as the Nation's central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To this end the Bureau conducts research and provides central national services in four broad program areas. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Radiation Research, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and com- merce. The Institute consists of an Office of Measurement Services and the following technical divisions: Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic and Molec- ular Physics—Radio Physics - —Radio Engineering -—Time and Frequency -—Astro- physics - —Cryogenics. -

Synthesis and Characterization of Diamond and Boron Phosphide
University of Rhode Island DigitalCommons@URI Open Access Dissertations 1972 Synthesis and Characterization of Diamond and Boron Phosphide K. P. Ananthanarayanan University of Rhode Island Follow this and additional works at: https://digitalcommons.uri.edu/oa_diss Recommended Citation Ananthanarayanan, K. P., "Synthesis and Characterization of Diamond and Boron Phosphide" (1972). Open Access Dissertations. Paper 690. https://digitalcommons.uri.edu/oa_diss/690 This Dissertation is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Dissertations by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. T-P 7'i7 3. S ])5 Aisc°' .:. 1 SYNTHESIS AND CHARACTERIZATION OF DIAMOND AND BORON PHOSPHIDE / BY K. P. ANANTHANARAYANAN A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN CHEMICAL ENGINEERING UNIVERSITY OF RHODE ISLAND 1972 DOCTOR OF PHILOSOPHY THESIS OF K. P. ANANTHANARAYANAN Approved: Thesis Committee: Dean of the Graduate School UNIVERSITY OF RHODE ISLAND 1972 ABSTRACT Semiconducting diamond has been synthesized from carbon-metal melts in a 600 ton tetrahedral anvil press at about 60 kbar and 1400°c. The experimental set up, pressure and temperature calibra- tions, and the growth region in the pressure-temperature regime are indicated. Micrographs of synthesized crystals are shown. The semiconducting properties of diamond, doped with boron, aluminum and titanium have been interpreted using the log R versus l/T curves. In boron-doped diamond ''high concentration" type im- purity conduction occurs and the activation energies vary from 0.15 eV to 0.30 eV. -

Other Than Radioactive Ores) Answering to a Description in Heading 2844 Or 2845 Are to Be Classified in Those Headings and in No Other Heading of the Tariff Schedule
)&f1y3X SECTION VI PRODUCTS OF THE CHEMICAL OR ALLIED INDUSTRIES VI-1 Notes 1. (a) Goods (other than radioactive ores) answering to a description in heading 2844 or 2845 are to be classified in those headings and in no other heading of the tariff schedule. (b) Subject to paragraph (a) above, goods answering to a description in heading 2843 or 2846 are to be classified in those headings and in no other heading of this section. 2. Subject to note 1 above, goods classifiable in heading 3004, 3005, 3006, 3212, 3303, 3304, 3305, 3306, 3307, 3506, 3707 or 3808 by reason of being put up in measured doses or for retail sale are to be classified in those headings and in no other heading of the tariff schedule. 3. Goods put up in sets consisting of two or more separate constituents, some or all of which fall in this section and are intended to be mixed together to obtain a product of section VI or VII, are to be classified in the heading appropriate to that product, provided that the constituents are: (a) Having regard to the manner in which they are put up, clearly identifiable as being intended to be used together without first being repacked; (b) Entered together; and (c) Identifiable, whether by their nature or by the relative proportions in which they are present, as being complementary one to another. Additional U.S. Notes 1. In determining the amount of duty applicable to a solution of a single compound in water subject to duty in this section at a specific rate, an allowance in weight or volume, as the case may be, shall be made for the water in excess of any water of crystallization which may be present in the undissolved compound. -

Ultrahigh Thermal Conductivity in Isotope-Enriched Cubic Boron Nitride
Ultrahigh thermal conductivity in isotope-enriched cubic boron nitride The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Chen, Ke et al. "Ultrahigh thermal conductivity in isotope-enriched cubic boron nitride." Science 367, 6477 (January 2020): 555-559 © 2020 The Authors As Published http://dx.doi.org/10.1126/science.aaz6149 Publisher American Association for the Advancement of Science (AAAS) Version Author's final manuscript Citable link https://hdl.handle.net/1721.1/127819 Terms of Use Creative Commons Attribution-Noncommercial-Share Alike Detailed Terms http://creativecommons.org/licenses/by-nc-sa/4.0/ Ultrahigh thermal conductivity in isotope-enriched cubic boron nitride Ke Chen1*, Bai Song1*†‡,, Navaneetha K. Ravichandran2*, Qiye Zheng3§, Xi Chen4#, Hwijong Lee4, Haoran Sun5, Sheng Li6, Geethal Amila Gamage5, Fei Tian5, Zhiwei Ding1, Qichen Song1, Akash Rai3, Hanlin Wu6, Pawan Koirala6, Aaron J. Schmidt1, Kenji Watanabe7, Bing Lv6, Zhifeng Ren5, Li Shi4,8, David G. Cahill3, Takashi Taniguchi7, David Broido2†, and Gang Chen1† 1Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. 2Department of Physics, Boston College, Chestnut Hill, MA 02467, USA. 3Department of Materials Science and Engineering and Materials Research Laboratory, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA. 4Materials Science and Engineering Program, Texas Materials Institute, The University of Texas at Austin, Austin, TX 78712, USA 5Department of Physics and Texas Center for Superconductivity, University of Houston, Houston, TX 77204, USA. 6Department of Physics, The University of Texas at Dallas, Richardson, TX 75080, USA. 7National Institute for Materials Science, Namiki 1-1, Tsukuba, Ibaraki 305-0044, Japan. -

Thermoelectric Transports in Pristine and Functionalized Boron Phosphide Monolayers Min‑Shan Li1,3, Dong‑Chuan Mo2,3 & Shu‑Shen Lyu2,3*
www.nature.com/scientificreports OPEN Thermoelectric transports in pristine and functionalized boron phosphide monolayers Min‑Shan Li1,3, Dong‑Chuan Mo2,3 & Shu‑Shen Lyu2,3* Recently, a new monolayer Group III–V material, two‑dimensional boron phosphide (BP), has shown great potential for energy storage and energy conversion applications. We study the thermoelectric properties of BP monolayer as well as the efect of functionalization by frst‑principles calculation and Boltzmann transport theory. Combined with a moderate bandgap of 0.90 eV and ultra‑high carrier mobility, a large ZT value of 0.255 at 300 K is predicted for two‑dimensional BP. While the drastically reduced thermal conductivity in hydrogenated and fuorinated BP is favored for thermoelectric conversion, the decreased carrier mobility has limited the improvement of thermoelectric fgure of merit. Termoelectric material, which can directly convert waste heat into electricity, provides a promising solution for global issues like energy crisis1–4. Te thermoelectric performance of a material can be characterized by a dimensionless fgure of merit: S2σT ZT = , (1) kel + kla where S is the Seebeck coefcient, σ is the electronic conductivity, kel and kla are the thermal conductivities con- tributed by electrons and phonons, respectively. To obtain high thermoelectric performance, a material generally needs to have high electrical conductivity σ, high Seebeck coefcient S and low thermal conductivity k at the same time5. While the inter-coupling of the electronic parameters has made the optimization of thermoelectric performance a great challenge, early studies suggest that lowering the dimension of materials appears to be an efective approach6–9.