Lactitol, a New Hydrogenated Lactose Derivative: Intestinal Absorption and Laxative Threshold in Normal Human Subjects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

“Polyols: a Primer for Dietetic Professionals” Is a Self-Study
1 “Polyols: A primer for dietetic professionals” is a self-study module produced by the Calorie Control Council, an accredited provider of continuing professional education (CPE) for dietetic professionals by the Commission on Dietetic Registration. It provides one hour of level 1 CPE credit for dietetic professionals. The full text of the module is in the notes section of each page, and is accompanied by summary points and/or visuals in the box at the top of the page. Directions for obtaining CPE are provided at the end of the module. 2 After completing this module, dietetic professionals will be able to: • Define polyols. • Identify the various types of polyols found in foods. • Understand the uses and health effects of polyols in foods. • Counsel clients on how to incorporate polyols into an overall healthful eating pattern. 3 4 Polyols are carbohydrates that are hydrogenated, meaning that a hydroxyl group replaces the aldehyde or ketone group found on sugars. Hydrogenated monosaccharides include erythritol, xylitol, sorbitol, and mannitol. Hydrogenated disaccharides include lactitol, isomalt, and maltitol. And hydrogenated starch hydrolysates (HSH), or polyglycitols (a wide range of corn syrups and maltodextrins), are formed from polysaccharides (Grabitske and Slavin 2008). 5 Nearly 54 percent of Americans are trying to lose weight, more than ever before. Increasingly, they are turning toward no- and low-sugar, and reduced calorie, foods and beverages to help them achieve their weight loss goals (78% of Americans who are trying to lose weight) (CCC 2010). Polyols, found in many of these foods, are becoming a subject of more interest. 6 They are incompletely digested , therefore are sometimes referred to as “low- digestible carbohydrates.” Polyols are not calorie free, as there is some degree of digestion and absorption of the carbohydrate. -

Study of the Partial and Exhaustive Intramolecular Dehydration of D-Sorbitol
Available online at http://journal-of-agroalimentary.ro Journal of Journal of Agroalimentary Processes and Agroalimentary Processes and Technologies 2013, 19(2), 259-270 Technologies Study of the partial and exhaustive intramolecular dehydration of D-sorbitol Ileana Cocan *, Monica Viorica Negrea, Ionel Vasile Jianu ”Dunărea de Jos” University of Galati, Faculty of Food Science and Engineering, Domnească Street, 47, RO-800008, Galati, Romania Received: 07 February 2013; Accepted: 09 March 2013 ______________________________________________________________________________________ Abstract Sweetener, humectants, sequestrant, texturizer, stabilizer, bulking agent, D-sorbitol represents a leading product in the processing of the polysorbate class. The scope of this work is to obtain details on the preparation and accession of D-sorbitol mono- and dianhydride as a method of chemical protection of primary and secondary hydroxyl functional groups (1:4) (3:6) as part of the strategy of controlled processing of polysorbates containing “homogeneous” polyoxyethylenic chains (n = 3, 6, 9, 18). This work is investigating the dependence of yield of internal dehydration (partial and/or exhaustive, direct of D-sorbitol) on temperature (100-160°C and 120-200°C , respectively ) and duration (5–35 minutes and 10–100 minutes , respectively ) for the processing of 1,4-sorbitan and 2,5-isosorbide. The evolution of the hydroxyl number (mg KOH/g D-sorbitol) (mg KOH/g 1,4-sorbitan) related to the theoretical (initial) value was followed and optimal values for the yield and dehydration parameters were established. Also in this work, the mathematical modeling of the dependence curves experimentally registered is realized. Keywords : izosorbide, D-sorbitol, D-glucitol, D-sorbit, 1,4-sorbitan ______________________________________________________________________________________ 1. -

Sorbitol Malabsorption 1/2
Internal Medicine and Gastroenterology Clinic Dres. Mares,M.Hanig,Mares, Hanig, Rambow, S.Blau, Blau, M.Seip, Hanig, Seip, A.Borchers,Blau,Kirchner, Hochstr. Hochstr. Hochstr. 43, 60313 43, 43, 60313 60313 Frankfurt/Main, Frankfurt/M., Frankfurt/M., www.gastroenterologie-ffm.de www.gastroenterologie-ffm.de www.gastroenterologie-ffm.de Nutrition hints for sorbitol malabsorption 1/2 Sorbitol intolerance Sorbitol incompatibility 1. What does this mean for nutrition? Avoid sorbitol as a sweetener and foods high in sorbitol. Small amount of sorbitol can often be tolerated (at most 10-10 g per day, sometimes less). In order to prevent general upper GI problems, easily digestible foods that do not cause gas are recommended. 2. Key points about foods – Avoid sorbitol and foods containing sorbitol – easily digestible food that does not cause gas 3. vegetables and fruits low in fibre (see 5) 4. Choice of foods The following foods are high in sorbitol and not suitable: – Sorbitol as sweetener: e.g. Sionon, Flarom, diabetic sweetener – Dietetic foods produced with sorbitol: for example, diabetic marmalades, diabetic sweets, diabetic baked goods – Types of fruit which have a naturally high sorbitol content: Apples, pears, cherries, prunes, plums, dates fruits with seeds, such as mirabelles, apricots, nectarines, and all fruit syrups made from these types of fruits – Types of fruit which have a naturally low sorbitol content: Berry fruits such as strawberries, raspberries, blackberries, blueberries, currants, gooseberries, citrus fruits, bananas, pineapples, kiwis – Sorbitol as a coating for: sultanas, raisins, and dried fruit or candied fruit – Sorbitol in sweets: chewing gum, jelly babies, jelly fruits, candies, chocolate bars, filled wafers, chocolate, etc. -

Copyrighted Material
Index Abbreviations receptor sites 202, 211 weak 122 amino acids, Table 500–1 muscarinic 413 weak, pH calculation 147–8 nucleic acids 551 nicotinic 413 Acid–base nucleotides, Table 551 as quaternary ammonium salt 202 catalysis, enzymes 516 peptides and proteins 504 Acetylcholinesterase equilibria 121 phosphates and diphosphates 277 enzyme mechanism 519–21 interactions, predicting 155–7 structural, Table 14 hydrolysis of acetylcholine 279 Acidic reagents, Table 157 ACE (angiotensin-converting enzyme) inhibitors 279 Acidity enzyme action 532 Acetyl-CoA carboxylase, in fatty acid acidity constant 122 inhibitors 532 biosynthesis 595 bond energy effects 125 Acetal Acetyl-CoA (acetyl coenzyme A) definition 121 in etoposide 233 as acylating agent 262 electronegativity effects 125 formation 229 carboxylation to malonyl-CoA 595, hybridization effects 128 polysaccharides as polyacetals 232 609 inductive effects 125–7 as protecting group 230 Claisen reaction 381 influence of electronic and structural Acetal and ketal enolate anions 373 features 125–34 cyclic, as protecting groups 481 in Krebs cycle 585 and leaving groups, Table 189 groups in sucrose 231 from β-oxidation of fatty acids 388 pKa values 122–5 Acetaldehyde, basicity 139 as thioester 262, 373 resonance / delocalization effects 129–34 Acetamide, basicity 139 Acetylene, bonding molecular orbitals 31 Acidity (compounds) Acetaminophen, see paracetamol Acetylenes, acidity 128 acetone 130 Acetoacetyl-CoA, biosynthesis from N-Acetylgalactosamine, in blood group acetonitrile 365 acetyl-CoA 392 -

Dental and Metabolic Effects of Lactitol in the Diet of Laboratory Rats
Downloaded from British Journal of Nutrition (1989), 61, 17-24 17 https://www.cambridge.org/core Dental and metabolic effects of lactitol in the diet of labolratory rats BY T. H. GRENBY AND A. PHILLIPS Department of Oral Medicine and Pathology, United Medical and Dental Schools, Guy’s Hospital, London SEI 9RT . IP address: (Received 17 May 1988 - Accepted 30 August 1988) 170.106.35.93 I. Because so little is known about the properties of lactitol as a possible alternative bulk sweetener to sucrose, it was tested in two large-scale experiments in laboratory rats. Matched groups of caries-active Osborne-Mendel rats were fed.on uniform diets containing lactitol and compared with a sucrose control in both experiments, plus a xylitol control in the first experiment. , on 2. In the early stages of the experiments weight gains and food utilization were better on the Sucrose than on 26 Sep 2021 at 05:12:03 the lactitol regimens. Body-fat storage was higher on the sucrose than on the polyol regimens. 3. At the end of 8 weeks the mandibular molars were examined for dental plaque accumulation and dental caries. The dental caries scores when 160 g sucrose/kg in the diet was replaced by lactitol were lower by a highly significant margin, bringing them down to the same low level as those on a 160 g xylitol/kg regimen. 4. Testing lactitol in a manufactured food product, shortbread biscuits, in comparison with ordinary sucrose biscuits, showed differences in plaque scores (significant) and caries levels (highly significant), with 60 % fewer lesions on the lactitol regimen. -

Polyols Have a Variety of Functional Properties That Make Them Useful Alternatives to Sugars in Applications Including Baked Goods
Polyols have a variety of functional properties that make them useful alternatives to sugars in applications including baked goods. Photo © iStockphoto.com/Synergee pg 22 09.12 • www.ift.org BY LYN NABORS and THERESA HEDRICK SUGAR REDUCTION WITH Polyols Polyols are in a unique position to assist with reduced-sugar or sugar-free reformulations since they can reduce calories and complement sugar’s functionality. ugar reduction will be an important goal over the of the product’s original characteristics may still be main- next few years as consumers, government, and in- tained with the replacement of those sugars by polyols. Sdustry alike have expressed interest in lower-calorie In addition, excellent, good-tasting sugar-free products and lower-sugar foods. The 2010 Dietary Guidelines for can be developed by using polyols. Polyols are in a unique Americans put a strong emphasis on consuming fewer position to assist with reduced-sugar or sugar-free refor- calories and reducing intake of added sugars. The In- mulations; since they are only partially digested and ab- stitute of Medicine (IOM) held a public workshop in sorbed, they can reduce calories and complement sugar’s November 2010 to discuss ways the food industry can functionality. Polyols provide the same bulk as sugars and use contemporary and innovative food processing tech- other carbohydrates. Additionally, polyols have a clean, nologies to reduce calorie intake in an effort to reduce sweet taste, which is important since consumers are not and prevent obesity, and in October 2011 recommended likely to sacrifice taste for perceived health benefits. Poly- front-of-package labeling that includes rating the product ols have a host of other functional properties that make based on added sugars content. -

Sweet Sensations by Judie Bizzozero | Senior Editor
[Confections] July 2015 Sweet Sensations By Judie Bizzozero | Senior Editor By R.J. Foster, Contributing Editor For many, terms like “reduced-sugar” or “sugar-free” do not go with the word “candy.” And yet, the confectionery industry is facing growing demand for treats that offer the taste people have grown to love without the adverse health effects they’re looking to avoid. Thankfully, there is a growing palette of ingredients from which candy makers can paint a new picture of sweetness that will be appreciated by the even most discerning of confectionery critics. SUGAR ALCOHOLS Also referred to as polyols, sugar alcohols are a common ingredient in reduced-sugar and sugar-free applications, especially confections. Funny thing, they’re not sugars or alcohols. Carbohydrate chains composed of monomeric, dimeric and polymeric units, polyols resemble both sugars and alcohols, but do not contain an ethanol molecule. All but two sugar alcohols are less sweet than sugar. Being only partially digestible, though, replacing a portion of a formulation’s sugar with a sugar alcohol reduces total calories without losing bulk (which can occur when replacing sugar with high-intensity sweeteners). Unique flavoring, texturizing and moisture-controlling effects also make polyols well-suited for confectionery products. Two very common and very similar monomeric polyols are sorbitol and mannitol. Present in a variety of fruits and vegetables, both are derived from products of cornstarch hydrolysis. Sorbitol is made via hydrogenation of glucose, which is why sorbitol is sometimes referred to as glucitol. Mannitol is created when fructose hydrogenation converts fructose into mannose, for which the final product, mannitol, is named. -

Lactitol, Bulk Sweetener for Sugar Free and Reduced Calories Hard
Sugar Free Dental Properties Lactitol is noncariogenic. It is not fermented by the oral micro flora, so its consumption does not lead to Lactitol, Bulk Sweetener for the formation of acids that deminer- alize the tooth enamel. Also, the building up of tooth plaque is much less for lactitol-containing hard can- dies when compared to sugar. Its Sugar Free and Reduced noncariogenic properties have been shown in various clinical trials (Grenby and Desai, 1988; Grenby, 1989; Grenby and Phillips, 1989; Grenby et al., 1989; van der Calories Hard Candy Hoeven, 1986). REGULATORY ASPECTS A self-affirmation petition for the Generally Recognized as Safe status of lactitol, submitted by Purac, was his paper will discuss the prop- intense sweetener like aspartame or accepted for filing by the Food and Terties, regulatory aspects, and acesulfame-K. The taste, sweetening power, and Drug Administration in September applications of lactitol in hard can- 1993. The safety of lactitol has been dies. Lactitol is a disaccharide sugar profile of such sweetener combina- tions are very close to those of substantiated by numerous animal alcohol made from lactose by cat- and human studies. This safety alytic hydrogenation. sucrose. Its clean sweet taste allows a superb flavor release. research has been reviewed by sev- eral international authoritative bod- BENEFITS OF LACTITOL Reduced Calories ies (JEFCA, 1983; EEC, 1984). The Taste The FDA allows the use of a self- joint FAO/WHO Committee on Lactitol has a clean, sweet, sugar- determined value of 2.0 kilocalorie Food Additives has approved lacti- like taste without an aftertaste. The per gram for lactitol. -

CHM 224 Test 2 Chapters 13, 14, Organometallics, 20 NAME
CHM 224 NAME: Test 2 Chapters 13, 14, organometallics, 20 1. Answer the following 3 questions: A. Brandy is 60% alcohol. What is its proof? B. This alcohol can be created by heating wood chips: C. This alcohol is referred to as "rubbing alcohol": 2. The three compounds below have nearly identical molecular weights. Arrange them according to their expected boiling points from highest >>> lowest. OH OH O A B C 3. Match the pKa values with the compounds provided: pKa's = 7.2, 8.0, 10.3 OH OH OH NO2 CH3 CN 4. What is the expected major product of the following reaction? 1. 1 equivalent BH3•THF 2. NaOH, H2O2 5. Which of the following compounds is expected to fail to react with KMnO4 (may be more than one)? A. 1-methylcyclopentanol B. 2-methyl-3-hexanol C. 4-ethyl-4-heptanol D. 3-bromo-1-butanol 6. Provide the IUPAC name for the following compound: OH Br 7. Which ONE of the following statements is true? A. ethers are generally water soluble, flammable, and reactive with strong bases B. ethers are generally water insoluble, not flammable, and reactive with strong acids C. ethers are generally water soluble, flammable, and reactive with strong bases D. ethers are generally water insoluble, flammable, and reactive with strong acids 8. Provide a synthetic route for the synthesis of tert-butyl isopropyl ether using the Williamson ether synthesis from an appropriate starting alcohol and alkyl halide. 9. Which one of the following alkenes will form the epoxide below upon treatment with a peroxyacid? O H CH2CH3 H3C CH3 A B C D 10. -

Pichia Process Optimization by Methanol/Sorbitol Co-Feeding – Patrick Fickers - University of Liege
Short Communication Journal of Applied Microbiology and Biochemistry 2020 Vol.4 No.4 Applied Microbiology 2016- Pichia process optimization by methanol/sorbitol co-feeding – Patrick Fickers - University of Liege Patrick Fickers University of Liege Abstract Recombinant protein production driven by AOX1 promoter is at this stage. During the second step, so as to extend biomass challenged by a high oxygen demand and warmth production, production and de-repress the methanol metabolic machinery, a especially in large-scale bioreactor. A reliable solution depends glycerol-limited fed-batch procedure is initiated. This phase on a methanol/sorbitol co-feeding strategy during the induction leads to gradual de-repression of the enzymes necessary for the phase. during this work, transient continuous cultures were first dissimilation of methanol and reduces the time necessary for performed to quantitatively assess the advantages of a the cells to adapt to growth on methanol. Finally, recombinant methanol/sorbitol co-feeding process with a P. pastoris Mut+ protein expression is typically induced by feeding methanol strain bearing a pAOX1-lacZ construct served as a reporter because the sole carbon source. gene. Our results demonstrated that cell-specific oxygen consumption (qO2) might be reduced by decreasing the A fermentation guideline for both Mut+ and Muts strains of P. methanol fraction within the feeding media. Optimal pAOX1 pastoris is available from Invitrogen . Stratton et al. devised a induction was achieved and maintained within the range of protocol for P. pastoris high cell-density fermentation, during 0.45~0.75 C-mol/C-mol of methanol fraction. additionally, the which detailed procedures are provided. -

Enhanced Protein Production by Sorbitol Co-Feeding with Methanol in Recombinant Pichia Pastoris Strains
Biotechnology and Bioprocess Engineering 22: 767-773 (2017) pISSN 1226-8372 DOI 10.1007/s12257-017-0011-9 eISSN 1976-3816 RESEARCH PAPER Enhanced Protein Production by Sorbitol Co-feeding with Methanol in Recombinant Pichia pastoris Strains Li Chen, Ali Mohsin, Ju Chu, Yingping Zhuang, Yamei Liu, and Meijin Guo Received: 9 January 2017 / Revised: 8 May 2017 / Accepted: 29 August 2017 © The Korean Society for Biotechnology and Bioengineering and Springer 2017 Abstract Pichia pastoris strains carrying 1, 6, 12, and 18 Keywords: sorbitol, co-feeding, multi-copy, Pichia pastoris, copies of the porcine insulin precursor (PIP) gene, were porcine insulin precursor employed to investigate the effects of sorbitol co-feeding with methanol on the physiology of the strains. Multicopy clones of the methylotrophic yeast were generated to vary 1. Introduction the PIP gene dosage and recombinant proteins. Elevated gene dosage increased levels of the recombinant PIP protein The methylotrophic yeast, Pichia pastoris, has been when methanol served as the sole carbon and energy developed to be a highly successful system for large-scale source i.e., an increase of 1.9% for a strain carrying 1 copy, production of functionally active recombinant proteins 42.6% for a strain carrying 6 copies, 34.7% for a strain [1,2]. As a yeast, it is a unique system which has many carrying 12 copies and 80.9% for a strain carrying 18 advantages e.g., its ability to produce foreign proteins at copies, respectively (using sorbitol co-feeding with methanol high levels using one of the strongest and highly regulated during the induction phase). -
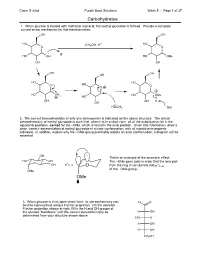
5.01 Carbohydrates Answers.Cdx
Chem S-20ab Purple Book Solutions Week 5 - Page 1 of 37 Carbohydrates 1. When glucose is treated with methanol and acid, the methyl glucoside is formed. Provide a complete curved-arrow mechanism for this transformation: OH OH HO CH OH, H+ HO O 3 O H+ HO OH HO OMe OH OH OH OH OH HO HO O HO O O HO OH HO OMe 2 HO OH OH H OH HOCH3 Sol 2. The correct stereochemistry of only one stereocenter is indicated on the above structure. The actual stereochemistry of methyl glucoside is such that, when it is in a chair form, all of the substituents fall in the equatorial positions, except for the –OMe, which is found in the axial position. Given this information, draw a clear, correct representation of methyl glucoside in a chair conformation, with all substituents properly indicated. In addition, explain why the –OMe group preferably adopts an axial conformation; a diagram will be essential. OH This is an example of the anomeric effect. HO OH The –OMe goes axial in order that the lone pair O * OH C–O O fromtheringOcandonateinto *C–O of the –OMe group. OMe OMe 3. When glucose is in its open-chain form, its stereochemistry can H O best be represented using a Fischer projection. On the skeleton Fischer projection shown at right, fill in the H and OH groups of the glucose "backbone" with the correct stereochemistry as H OH determined from your structure shown above. HO H H OH H OH CH2OH Chem S-20ab Purple Book Solutions Week 5 - Page 2 of 37 Carbohydrates: Mechanisms 1.