To Scream Or to Listen? Prey Detection and Discrimination in Animal-Eating Bats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -
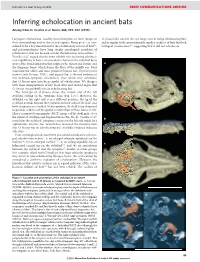
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

BIO 313 ANIMAL ECOLOGY Corrected
NATIONAL OPEN UNIVERSITY OF NIGERIA SCHOOL OF SCIENCE AND TECHNOLOGY COURSE CODE: BIO 314 COURSE TITLE: ANIMAL ECOLOGY 1 BIO 314: ANIMAL ECOLOGY Team Writers: Dr O.A. Olajuyigbe Department of Biology Adeyemi Colledge of Education, P.M.B. 520, Ondo, Ondo State Nigeria. Miss F.C. Olakolu Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. Mrs H.O. Omogoriola Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. EDITOR: Mrs Ajetomobi School of Agricultural Sciences Lagos State Polytechnic Ikorodu, Lagos 2 BIO 313 COURSE GUIDE Introduction Animal Ecology (313) is a first semester course. It is a two credit unit elective course which all students offering Bachelor of Science (BSc) in Biology can take. Animal ecology is an important area of study for scientists. It is the study of animals and how they related to each other as well as their environment. It can also be defined as the scientific study of interactions that determine the distribution and abundance of organisms. Since this is a course in animal ecology, we will focus on animals, which we will define fairly generally as organisms that can move around during some stages of their life and that must feed on other organisms or their products. There are various forms of animal ecology. This includes: • Behavioral ecology, the study of the behavior of the animals with relation to their environment and others • Population ecology, the study of the effects on the population of these animals • Marine ecology is the scientific study of marine-life habitat, populations, and interactions among organisms and the surrounding environment including their abiotic (non-living physical and chemical factors that affect the ability of organisms to survive and reproduce) and biotic factors (living things or the materials that directly or indirectly affect an organism in its environment). -

Origin and Beyond
EVOLUTION ORIGIN ANDBEYOND Gould, who alerted him to the fact the Galapagos finches ORIGIN AND BEYOND were distinct but closely related species. Darwin investigated ALFRED RUSSEL WALLACE (1823–1913) the breeding and artificial selection of domesticated animals, and learned about species, time, and the fossil record from despite the inspiration and wealth of data he had gathered during his years aboard the Alfred Russel Wallace was a school teacher and naturalist who gave up teaching the anatomist Richard Owen, who had worked on many of to earn his living as a professional collector of exotic plants and animals from beagle, darwin took many years to formulate his theory and ready it for publication – Darwin’s vertebrate specimens and, in 1842, had “invented” the tropics. He collected extensively in South America, and from 1854 in the so long, in fact, that he was almost beaten to publication. nevertheless, when it dinosaurs as a separate category of reptiles. islands of the Malay archipelago. From these experiences, Wallace realized By 1842, Darwin’s evolutionary ideas were sufficiently emerged, darwin’s work had a profound effect. that species exist in variant advanced for him to produce a 35-page sketch and, by forms and that changes in 1844, a 250-page synthesis, a copy of which he sent in 1847 the environment could lead During a long life, Charles After his five-year round the world voyage, Darwin arrived Darwin saw himself largely as a geologist, and published to the botanist, Joseph Dalton Hooker. This trusted friend to the loss of any ill-adapted Darwin wrote numerous back at the family home in Shrewsbury on 5 October 1836. -

103 the EVOLUTION of BATSI Leigh Van Valen Department Of
103 THE EVOLUTIONOF BATSI Leigh Van Valen Department of Biology University of Chicago 1103 East 57th Street Chicago, Illinois 60637 Received August 30, 1978; June 8, L979 Abstract: I devel-op the first explicitly Justified phylogeny of the known farnil-les of bats, usj.ng all available characters. This phylogeny permits the adaptive evolution of the order to be outlined. Two main clades emerge within the Microchiroptera and are cal-led new infraorders, Vespertilionia and Phyllostomatia. In each infraorder there is a series of grades of progressively stronger fi-ight, and there is a radiation of diet within the Phyllostomatia. Parallel evolution is extensive. Most grades stlll exist, presumably by a poorly understood partitioning of the resource space. The Megachiroptera nay have originated in the l-ate Ollgocene or early Miocene from surviving mernbers of the Eochiroptera (new suborder). The Kerivoul,ldae, Myzopodidae, Thyropteridae, and Furlpterldae are placed in the Natalidae, and the lcaronycterididae in the PaLaeochiropterygidae. The features of an ancestral bat are predicted. Bat origins are poorly known but may be found in Paleocene members of the Adapisoricidae. ,r** Bats constitute the second largest order of marnmalsand have a readily decipherable adaptive history, at least compared with the Rodentia and Insectivora. Nevertheless, there is no treatment of this adaptive hlstory nor even a general phyl-ogeny, which must form its foundation. I noticed this l-ack when revising a course on the paleobiology of mauunalsand undertook to remedy it. Although I sttll lack much familiarity with bats, the resul-t may be of more general interest. ORIGIN OF BATS One may hypothesize ttrat bats dld originate, but it is harder to go beyond this. -

Messel Pit – Wikipedia Germany
03/08/2018 Messel pit - Wikipedia Coordinates: 49°55′03″N 8°45′24″E Messel pit The Messel Pit (German: Grube Messel) is a disused quarry near the Messel Pit Fossil Site village of Messel, (Landkreis Darmstadt-Dieburg, Hesse) about 35 km (22 mi) southeast of Frankfurt am Main, Germany. Bituminous shale UNESCO World Heritage site was mined there. Because of its abundance of fossils, it has significant geological and scientific importance. After almost becoming a landfill, strong local resistance eventually stopped these plans and the Messel Pit was declared a UNESCO World Heritage site on 9 December 1995. Significant scientific discoveries are still being made and the site has increasingly become a tourist site as well. Contents Location Darmstadt-Dieburg, History Hesse, Germany Depositional characteristics Criteria Natural: (viii) Volcanic gas releases Reference 720bis (http://whc.unesco. Fossils org/en/list/720bis) Mammals Inscription 1995 (19th Session) Birds Reptiles Extensions 2010 Fish Area 42 ha (4,500,000 sq ft) Insects Plants Buffer zone 22.5 ha (2,420,000 sq ft) Access Coordinates 49°55′03″N 8°45′24″E See also References External links History Brown coal and later oil shale was actively mined from 1859. The pit first became known for its wealth of fossils around 1900, but serious scientific excavation only started around the 1970s, when falling oil prices made the quarry uneconomical. Commercial oil shale mining ceased in 1971 and a cement factory built in the quarry failed the following year. The land was slotted for use as a landfill, but the plans came to nought and the Hessian state bought the site in 1991 to secure scientific access. -

Integrated Fossil and Molecular Data Reconstruct Bat Echolocation
Integrated fossil and molecular data reconstruct bat echolocation Mark S. Springer†‡, Emma C. Teeling†§, Ole Madsen¶, Michael J. Stanhope§ʈ, and Wilfried W. de Jong¶** †Department of Biology, University of California, Riverside, CA 92521; §Queen’s University of Belfast, Biology and Biochemistry, 97 Lisburn Road, Belfast BT9 7BL, United Kingdom; ¶Department of Biochemistry, University of Nijmegen, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands; ʈBioinformatics, SmithKline Beecham Pharmaceuticals, 1250 South Collegeville Road, UP1345, Collegeville, PA 19426; and **Institute for Biodiversity and Ecosystem Dynamics, 1090 GT Amsterdam, The Netherlands Edited by David Pilbeam, Harvard University, Cambridge, MA, and approved March 26, 2001 (received for review November 21, 2000) Molecular and morphological data have important roles in illumi- tulates a sister-group relationship between flying lemurs and bats nating evolutionary history. DNA data often yield well resolved (6). Although some authors have questioned bat monophyly phylogenies for living taxa, but are generally unattainable for based on evidence from the penis and nervous system (7, 8), the fossils. A distinct advantage of morphology is that some types of bulk of morphological data supports bat monophyly (9). Among morphological data may be collected for extinct and extant taxa. living Chiroptera, morphological data provide strong support for Fossils provide a unique window on evolutionary history and may the reciprocal monophyly of megachiropterans (Old World fruit preserve combinations -

Little Brown Bat (Myotis Lucifugus) BAC Library
Proposal for the construction of a Little Brown Bat (Myotis Lucifugus) BAC Library Russell Ray and Mario Capecchi, Dept of Human Genetics, University of Utah, Salt Lakc City, Utah. The importance of Myotis lucifugus to biomedical and biological research Bats have acquired a number of evolutionary innovations in adapting to their particular niche. Microbats such as Myotis lucifugus have a number of interesting features including heavily modified limbs, the ability to echolocate, precise control over both thermoregulation and gestation, and an unexpectedly long life span. Many of the unique structures in bats have not arisen de novo, but are highly modified homologous structures found among other mammals. A good example is the bat forelimb. Despite the gross changes in morphology required for flight, the bat forelimb has maintained the basic pentadactyl skeletal elements in its wing. However, these bones have changed dramatically, having been elongated and reduced in girth, while tissue that is normally eliminated between the digits persists to form the different patagium (membranes forming the wings)(Adams 1992). The development of such marked evolutionary innovations is of great interest to our laboratory and many others. Understanding limb development in bats will be informative towards the basic mechanisms important in limb length determination and in the fate of interdigit tissues, both of which are key in human limb development and are often corrupted in limb malformations. One important aspect to an understanding of the mechanisms used in evolution is the differential regulation of genes held in common among mammals. An obvious but untested hypothesis is that elaboration of homologous structures in bats resulted from changes in regulatory elements for genes governing these structures. -

Evolution of Body Mass in Bats: Insights from a Large Supermatrix Phylogeny
Journal of Mammalian Evolution https://doi.org/10.1007/s10914-018-9447-8 ORIGINAL PAPER Evolution of Body Mass in Bats: Insights from a Large Supermatrix Phylogeny Reyna Leticia Moyers Arévalo1 & Lucila I. Amador1 & Francisca C. Almeida2 & Norberto P. Giannini1,3,4 # Springer Science+Business Media, LLC, part of Springer Nature 2018 Abstract Bats are atypical small mammals. Size is crucial for bats because it affects most aerodynamic variables and several key echolocation parameters. In turn, scaling relationships of both flight and echolocation have been suggested to constrain bat body size evolution. Previous studies have found a large phylogenetic effect and the inclusion of early Eocene fossil bats contributed to recover idiosyncratic body size change patterns in bats. Here, we test these previous hypotheses of bat body size evolution using a large, comprehensive supermatrix phylogeny (+800 taxa) to optimize body size and examine changes reconstructed along branches. Our analysis provides evidence of rapid stem phyletic nanism, an ancestral value stabilized at 12 g for crown-clade Chiroptera followed by backbone stasis, low-magnitude changes inside established families, and massive body size increase at accelerated rate in pteropodid subclades. Total variation amount explained by pteropodid subclades was 86.3%, with most changes reconstructed as phyletic increases but also apomorphic decreases. We evaluate these macroevolutionary patterns in light of the constraints hypothesis, and in terms of both neutral and adaptive evolutionary models. The reconstructed macroevo- lution of bat body size led us to propose that echolocation and flight work as successive, nested constraints limiting bat evolution along the body size scale. Keywords Chiroptera . -

Harrison:Layout 1.Qxd
Acta Chiropterologica, 12(1): 1–18, 2010 PL ISSN 1508-1109 © Museum and Institute of Zoology PAS doi: 10.3161/150811010X504554 Late Middle Eocene bats from the Creechbarrow Limestone Formation, Dorset, southern England with description of a new species of Archaeonycteris (Chiroptera: Archaeonycteridae) DAVID L. HARRISON1, 3 and JEREMY J. HOOKER2 1Harrison Institute, Centre for Systematics and Biodiversity Research, Bowerwood House, St. Botolph’s Road, Sevenoaks, Kent TN13 3AQ, Great Britain 2Department of Palaeontology, The Natural History Museum, Cromwell Road, London, SW7 5BD, Great Britain 3Corresponding author: E-mail: [email protected] Isolated teeth of Chiroptera from the Creechbarrow Limestone Formation of late Middle Eocene age are reported. Five distinct chiropteran taxa are present. A new species of Archaeonycteris is described, representing the last survivor of this archaic genus. Two rhinolophoid species include the hipposiderid Pseudorhinolophus schlosseri and Rhinolophidae gen. et sp. indet. Vespertilionoid bats are represented by one species Stehlinia quercyi. A single trigonid represents a small species, which could have affinity with the genus Ageina. Key words: Late Middle Eocene, Archaeonycteris n.sp., Hipposideridae, Rhinolophidae, Vespertilionidae, Ageina INTRODUCTION Weid mann (2000: 126–127) provided an updated partial faunal list for the site, including a number of Rather little is known about bats in the British distinctively Bartonian species, such as Lo phio the - Tertiary, owing to the rarity of their remains in de- rium siderolithicum (Perissodactyla) and Ailu ravus posits laid down in open environments. Below is stehlin schaubi (Rodentia) and revised the definition a summary of records to date. of the lautricense-siderolithicum zone. Hooker The oldest British bat is Eppsinycteris, a genus (1986: 241, figs. -

Messel Pit Fossil Site - 2017 Conservation Outlook Assessment (Archived)
IUCN World Heritage Outlook: https://worldheritageoutlook.iucn.org/ Messel Pit Fossil Site - 2017 Conservation Outlook Assessment (archived) IUCN Conservation Outlook Assessment 2017 (archived) Finalised on 09 November 2017 Please note: this is an archived Conservation Outlook Assessment for Messel Pit Fossil Site. To access the most up-to-date Conservation Outlook Assessment for this site, please visit https://www.worldheritageoutlook.iucn.org. Messel Pit Fossil Site SITE INFORMATION Country: Germany Inscribed in: 1995 Criteria: (viii) Site description: Messel Pit is the richest site in the world for understanding the living environment of the Eocene, between 57 million and 36 million years ago. In particular, it provides unique information about the early stages of the evolution of mammals and includes exceptionally well-preserved mammal fossils, ranging from fully articulated skeletons to the contents of stomachs of animals of this period. © UNESCO IUCN World Heritage Outlook: https://worldheritageoutlook.iucn.org/ Messel Pit Fossil Site - 2017 Conservation Outlook Assessment (archived) SUMMARY 2017 Conservation Outlook Good The conservation outlook for the Messel Pit Fossil Site is good in the long term. The World Heritage values of the site are well preserved and stable thanks to the adequate and improving system of protection and management. The current and potential threats are very low and are all included in the current management plan, which is in place since 2009. The threats are well identified and the risks for the site are adequately estimated and addressed. Current state and trend of VALUES Good Trend: Improving The site’s World Heritage values are associated with the fossil record of a unique Middle Eocene environment, including various communities of aquatic, terrestrial and aerial organisms. -

The Evolution and Development of Chiropteran Flight
University of New Hampshire University of New Hampshire Scholars' Repository Honors Theses and Capstones Student Scholarship Spring 2020 The Evolution and Development of Chiropteran Flight Emmaline Willis University of New Hampshire Follow this and additional works at: https://scholars.unh.edu/honors Part of the Developmental Biology Commons, Evolution Commons, Other Ecology and Evolutionary Biology Commons, and the Zoology Commons Recommended Citation Willis, Emmaline, "The Evolution and Development of Chiropteran Flight" (2020). Honors Theses and Capstones. 538. https://scholars.unh.edu/honors/538 This Senior Honors Thesis is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Honors Theses and Capstones by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. Willis 1 Emmaline Willis Zool Thesis Advisor- Bolker 9/25/19 The Evolution and Development of Chiropteran Flight Intro Flying is thought of as a reserved skill used by very few animals throughout history. These animals may be the birds outside your window, the pterodactyl in a children’s book, or the insects buzzing about on a hot day. There are also thousands of bats flapping around in the dark all around the world. Exploring the sky, bats are the only mammal capable of true powered flight (Gunnel and Simmons 2005). Bats are an extraordinarily diverse group of mammals. Chiroptera, the order name for this group of mammals, has over one thousand species (Hill, 2018). In fact, the number may be closer to 1,400 species (K.