103 the EVOLUTION of BATSI Leigh Van Valen Department Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -
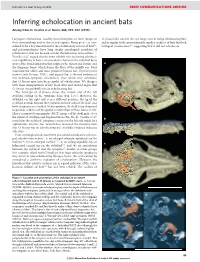
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

Taxonomy and Affinities of African Cenozoic Metatherians
Spanish Journal of Palaeontology 36 (2), 2021 https://doi.org/10.7203/sjp.36.2.20974 Sociedad Española de Paleontología ISSN 2255-0550 / eISSN 2660-9568 OPEN ACCESS RESEARCH PAPER Taxonomy and affi nities of african cenozoic metatherians Taxonomía y afi nidades de los metaterios cenozoicos africanos Vicente D. CRESPO & Francisco J. GOIN Abstract: The record of extinct African metatherians (Mammalia, Theria) is scanty, restricted Received: 20 January 2021 in time (Eocene–Miocene), and its taxonomy is still subject of debate. A review of all African Accepted: 24 May 2021 metatherians, or alleged metatherians, known up to now, led us to the recognition of only Published online: XXX three taxa referable to this group: (1) Kasserinotherium tunisiense (Peradectoidea?), from the early Eocene of Tunisia; (2) Peratherium africanum (Herpetotheriidae), from the early Oligocene of Egypt and Oman, and (3) an indeterminate Herpetotheriidae? from the early Corresponding author: Miocene of Uganda. Herpetotheriids probably reached Afro-Arabia from Europe in one Vicente D. Crespo or more dispersal waves since the early Oligocene. Kasserinotherium, on the contrary, [email protected] suggests an earlier (Paleocene) arrival from South America, judging from its alleged affi nities with South American and Australian taxa. Such a migration event (probably, Keywords: through a fi lter corridor such as the Rio Grande Rise-Walvis Ridge system in the South Mammalia Atlantic) may also explain the enigmatic presence of polydolopimorphian metatherians in Metatheria the Cenozoic of central Anatolia (Turkey). A more radical hypothesis is that all European (Eurasian?) Marsupialiformes have an ultimate origin in South America, from where they Africa dispersed via Africa by the Paleocene–earliest Eocene. -

Messel Pit – Wikipedia Germany
03/08/2018 Messel pit - Wikipedia Coordinates: 49°55′03″N 8°45′24″E Messel pit The Messel Pit (German: Grube Messel) is a disused quarry near the Messel Pit Fossil Site village of Messel, (Landkreis Darmstadt-Dieburg, Hesse) about 35 km (22 mi) southeast of Frankfurt am Main, Germany. Bituminous shale UNESCO World Heritage site was mined there. Because of its abundance of fossils, it has significant geological and scientific importance. After almost becoming a landfill, strong local resistance eventually stopped these plans and the Messel Pit was declared a UNESCO World Heritage site on 9 December 1995. Significant scientific discoveries are still being made and the site has increasingly become a tourist site as well. Contents Location Darmstadt-Dieburg, History Hesse, Germany Depositional characteristics Criteria Natural: (viii) Volcanic gas releases Reference 720bis (http://whc.unesco. Fossils org/en/list/720bis) Mammals Inscription 1995 (19th Session) Birds Reptiles Extensions 2010 Fish Area 42 ha (4,500,000 sq ft) Insects Plants Buffer zone 22.5 ha (2,420,000 sq ft) Access Coordinates 49°55′03″N 8°45′24″E See also References External links History Brown coal and later oil shale was actively mined from 1859. The pit first became known for its wealth of fossils around 1900, but serious scientific excavation only started around the 1970s, when falling oil prices made the quarry uneconomical. Commercial oil shale mining ceased in 1971 and a cement factory built in the quarry failed the following year. The land was slotted for use as a landfill, but the plans came to nought and the Hessian state bought the site in 1991 to secure scientific access. -

A Taxonomic Review of Hipposideros Halophyllus Hill and Yenbutra, 1984
A Taxonomic Review of Hipposideros halophyllus Hill and Yenbutra, 1984, Hipposideros ater Templeton, 1848, and Hipposideros cineraceus Blyth, 1853 (Chiroptera: Hipposideridae) in Thailand and Myanmar Bounsavane Douangboubpha A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Ecology (International Program) Prince of Songkla University 2008 Copyright of Prince of Songkla University i Thesis Title A Taxonomic Review of Hipposideros halophyllus Hill and Yenbutra, 1984, Hipposideros ater Templeton, 1848, and Hipposideros cineraceus Blyth, 1853 (Chiroptera: Hipposideridae) in Thailand and Myanmar Author Mr. Bounsavane Douangboubpha Major Program Ecology (International Program) Major Advisor Examining Committee: ………………………………………. .....………………………...Committee (Asst. Prof. Dr. Sara Bumrungsri) (Assoc. Prof. Dr. Kitichate Sridith) .....………………………...Committee Co-advisor (Dr. Chavalit Vidthayanon) ………………………………………. .....………………………...Committee (Assoc. Prof. Dr. Chutamas Satasook) (Asst. Prof. Dr. Sara Bumrungsri) ………………………………………. .....………………………...Committee (Dr. Paul J. J. Bates) (Assoc. Prof. Dr. Chutamas Satasook) The Graduate School, Prince of Songkla University, has approved this thesis as partial fulfillment of the requirements for the Master of Science Degree in Ecology (International Program). ………………………………………... (Assoc. Prof. Dr. Krerkchai Thongnoo) Dean of Graduate School ii Thesis Title A Taxonomic Review of Hipposideros halophyllus Hill and Yenbutra, 1984, Hipposideros ater Templeton, 1848, and -

To Scream Or to Listen? Prey Detection and Discrimination in Animal-Eating Bats
96 P.L. Jones et al. Fig. 4.1 Current phylogeny for bats (Jones and Teeling 2006 ). To the right of each family name, a Substrate Gleaner icon indicates that, in our opinion, this family is characterized by bat species that rely primarily on a gleaning strategy; all or most of which also take some prey by aerial hawk- ing. An Aerial Hawker icon indicates that the family consists of species most of which primarily use a hawking strategy but includes behaviorally fl exible species. Crasoenycteridae comprises a single behaviorally fl exible species. A Frugivore/Nectivore icon indicates a family comprised solely or partially of frugivorous and nectivorous species Each scenario, however, still suggests the same general story (Figure 4.1 ). That is, early laryngeal echolocating bats used powered fl ight, hunted animals, and took them in the air. The latter supposition is supported by the fact that most early fossil bats (~50 million years old) had wing designs suited for aerially hawking and not 4 Prey Detection and Discrimination in Animal-Eating Bats 97 like those of modern gleaners (Simmons and Geisler 1998 ; Safi et al. 2005 ). Therefore, we suppose that while something akin to gleaning may have characterized proto-bats and the very earliest of bats, this trait may have been subsequently lost, at least as a primary means of prey capture, as bats evolved more sophisticated laryngeal echolocation and longer, narrower wings, and then gleaning evolved inde- pendently again multiple times (Simmons and Geisler 1998 ) (Figure 4.1 ). However, while this idea is supported by fossil evidence (Simmons and Geisler 1998 ) and phylogenetic trait reconstructions (Safi et al. -

Integrated Fossil and Molecular Data Reconstruct Bat Echolocation
Integrated fossil and molecular data reconstruct bat echolocation Mark S. Springer†‡, Emma C. Teeling†§, Ole Madsen¶, Michael J. Stanhope§ʈ, and Wilfried W. de Jong¶** †Department of Biology, University of California, Riverside, CA 92521; §Queen’s University of Belfast, Biology and Biochemistry, 97 Lisburn Road, Belfast BT9 7BL, United Kingdom; ¶Department of Biochemistry, University of Nijmegen, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands; ʈBioinformatics, SmithKline Beecham Pharmaceuticals, 1250 South Collegeville Road, UP1345, Collegeville, PA 19426; and **Institute for Biodiversity and Ecosystem Dynamics, 1090 GT Amsterdam, The Netherlands Edited by David Pilbeam, Harvard University, Cambridge, MA, and approved March 26, 2001 (received for review November 21, 2000) Molecular and morphological data have important roles in illumi- tulates a sister-group relationship between flying lemurs and bats nating evolutionary history. DNA data often yield well resolved (6). Although some authors have questioned bat monophyly phylogenies for living taxa, but are generally unattainable for based on evidence from the penis and nervous system (7, 8), the fossils. A distinct advantage of morphology is that some types of bulk of morphological data supports bat monophyly (9). Among morphological data may be collected for extinct and extant taxa. living Chiroptera, morphological data provide strong support for Fossils provide a unique window on evolutionary history and may the reciprocal monophyly of megachiropterans (Old World fruit preserve combinations -

Little Brown Bat (Myotis Lucifugus) BAC Library
Proposal for the construction of a Little Brown Bat (Myotis Lucifugus) BAC Library Russell Ray and Mario Capecchi, Dept of Human Genetics, University of Utah, Salt Lakc City, Utah. The importance of Myotis lucifugus to biomedical and biological research Bats have acquired a number of evolutionary innovations in adapting to their particular niche. Microbats such as Myotis lucifugus have a number of interesting features including heavily modified limbs, the ability to echolocate, precise control over both thermoregulation and gestation, and an unexpectedly long life span. Many of the unique structures in bats have not arisen de novo, but are highly modified homologous structures found among other mammals. A good example is the bat forelimb. Despite the gross changes in morphology required for flight, the bat forelimb has maintained the basic pentadactyl skeletal elements in its wing. However, these bones have changed dramatically, having been elongated and reduced in girth, while tissue that is normally eliminated between the digits persists to form the different patagium (membranes forming the wings)(Adams 1992). The development of such marked evolutionary innovations is of great interest to our laboratory and many others. Understanding limb development in bats will be informative towards the basic mechanisms important in limb length determination and in the fate of interdigit tissues, both of which are key in human limb development and are often corrupted in limb malformations. One important aspect to an understanding of the mechanisms used in evolution is the differential regulation of genes held in common among mammals. An obvious but untested hypothesis is that elaboration of homologous structures in bats resulted from changes in regulatory elements for genes governing these structures. -

Evolution of Body Mass in Bats: Insights from a Large Supermatrix Phylogeny
Journal of Mammalian Evolution https://doi.org/10.1007/s10914-018-9447-8 ORIGINAL PAPER Evolution of Body Mass in Bats: Insights from a Large Supermatrix Phylogeny Reyna Leticia Moyers Arévalo1 & Lucila I. Amador1 & Francisca C. Almeida2 & Norberto P. Giannini1,3,4 # Springer Science+Business Media, LLC, part of Springer Nature 2018 Abstract Bats are atypical small mammals. Size is crucial for bats because it affects most aerodynamic variables and several key echolocation parameters. In turn, scaling relationships of both flight and echolocation have been suggested to constrain bat body size evolution. Previous studies have found a large phylogenetic effect and the inclusion of early Eocene fossil bats contributed to recover idiosyncratic body size change patterns in bats. Here, we test these previous hypotheses of bat body size evolution using a large, comprehensive supermatrix phylogeny (+800 taxa) to optimize body size and examine changes reconstructed along branches. Our analysis provides evidence of rapid stem phyletic nanism, an ancestral value stabilized at 12 g for crown-clade Chiroptera followed by backbone stasis, low-magnitude changes inside established families, and massive body size increase at accelerated rate in pteropodid subclades. Total variation amount explained by pteropodid subclades was 86.3%, with most changes reconstructed as phyletic increases but also apomorphic decreases. We evaluate these macroevolutionary patterns in light of the constraints hypothesis, and in terms of both neutral and adaptive evolutionary models. The reconstructed macroevo- lution of bat body size led us to propose that echolocation and flight work as successive, nested constraints limiting bat evolution along the body size scale. Keywords Chiroptera . -

Evolution and Paleoenvironment of Early Modern Vertebrates During the Paleogene Program and Abstracts
September 10‐13, 2019 Royal Belgian Institute of natural Sciences, Brussels, Belgium International symposium Evolution and Paleoenvironment of Early Modern Vertebrates during the Paleogene Program and abstracts PalEurAfrica Meeting 2019 In the framework of the PalEurAfrica research project (see http://www.paleurafrica.be), we are happy to invite you to the Royal Belgian Institute of Natural Sciences (RBINS) for an international symposium related to the evolution and paleoenvironment of early modern vertebrates during the Paleogene. This allows us to gather specialists who work on macro‐ and micropaleontology, bio‐ and isotope stratigraphy, paleoenvironment, paleogeography, and geology of Paleogene vertebrate bearing sites. This international meeting also celebrates the memory of one of our PalEurAfrica partners, Gregg Gunnell (1954‐2017), who tragically died unexpectedly in the middle of his career, having made significant contributions to our understanding of research on Paleogene vertebrate evolutionary history. Host committee Thierry Smith (Chair) – Royal Belgian Institute of Natural Sciences, Brussels, Belgium Thierry De Putter – Royal Museum for Central Africa, Tervuren, Belgium Stephen Louwye – Universiteit Gent, Gent, Belgium Johan Yans – Université de Namur, Namur, Belgium Matthew Borths – Duke University Lemur center, Durham, NC, USA Nancy Stevens – Ohio University, Athens, OH, USA Scientific committee Massimo Delfino – Università di Torino, Torino, Italy Gilles Escarguel – Université de Lyon, Lyon, France Annelise Folie – Royal Belgian Institute of Natural Sciences, Brussels, Belgium Emmanuel Gheerbrant – Museum national d’Histoire Naturelle, Paris, France Jason Head – University of Cambridge, Cambridge, UK Gerald Mayr – Senckenberg Forschungsinstitut und Naturmuseum, Frankfurt, Germany Florias Mees – Royal Museum for Central Africa, Tervuren, Belgium Ellen R. Miller – Wake Forest University, Winston‐Salem, NC, USA Adán Pérez‐García – Universidad Nacional de Educación a Distancia, Madrid, Spain Rajendra Singh Rana – H.N.B. -

New Miocene and Pliocene Megadermatids (Mammalia, Micro Chiroptera) from Australia, with Comments on Broader Aspects of Megadermatid Evolution
NEW MIOCENE AND PLIOCENE MEGADERMATIDS (MAMMALIA, MICRO CHIROPTERA) FROM AUSTRALIA, WITH COMMENTS ON BROADER ASPECTS OF MEGADERMATID EVOLUTION Suzar.nuJ.IIAND HAND S.J. 1996. New Miocene and Pliocene megadermatids (Mammalia, Microchiroptera) from Australia, with com- ments on broader aspects of megadermatid evolution. [Nouveaux M6gadermatid6s miocdnes et pliocbnes (Mammgliq, Microchiroptera) d'Australie;commentaires sur les aspects g6n6raux de l'6volution des M6gadermatid6sl. GEOBIOS' 29,3 :365-377. Villeurbanne le 30.06.1996. Manuscrit d6pos6 le 17.05.1993 ; accept6 d6finitivement le 10.01.1995. ABSTRACT - Macroderma malugara nov. sp., a new false vampire from the middle Miocene Gotham City Site of Riversleigh Station in northwestern Queensland, is described and three others discussed. Seven megadermatids are now known from the Australian fossil record. Morphological changes in the Australian Macroderma lineage are traced from the Oligo-Miocene to the present. In some Riversleigh deposits, there is evidence for sy'rnpatry of very differently-sized megadermatids. Riversleigh megadermatids provide an opportunity to trace an apparent trend to shorten the face in the Mairoclerma lineage flom the Oligo-Miocene to the present and to fufther document a tendency to gigantism in inde- pendent megadermatid lineages. KEY.WORDS : CHIROPTtrRA. NEW SPECIES, EVOLUTION, MIOCENE, PLIOCENE, AUSTRALIA RESUME - Macroderma malugara nov. sp.) un nouveau faux vampire du Miocdne moyen de Gotham City, Riversleigh Station, Queensland NW, est d6crit, et trois autres formes sont discut6es. Sept m6gadermatid6s fossiles sont maintenant connus d'Australie. Les modifications morphologiques dans la lign6e australienne de Macroderma sont retrac6es depuis l'Oligo-Miocbne jusqu'ir lActuel. Dans certains d6pdts de Riversleigh, la sSnnpatrie de m6gadermatid6s de diff6rentes tailles est 6vidente. -

New Early Eocene Mammalian Fauna from Western Patagonia, Argentina
Tejedor, M F; Goin, F J; Gelfo, J N; López, G; Bond, M; Carlini, A A; Scillato-Yané, G J; Woodburne, M O; Chornogubsky, L; Aragón, E; Reguero, M A; Czaplewski, N J; Vincon, S; Martin, G M; Ciancio, M R (2009). New Early Eocene mammalian fauna from western Patagonia, Argentina. American Museum Novitates, (3638):1-43. Postprint available at: http://www.zora.uzh.ch University of Zurich Posted at the Zurich Open Repository and Archive, University of Zurich. Zurich Open Repository and Archive http://www.zora.uzh.ch Originally published at: American Museum Novitates 2009, (3638):1-43. Winterthurerstr. 190 CH-8057 Zurich http://www.zora.uzh.ch Year: 2009 New Early Eocene mammalian fauna from western Patagonia, Argentina Tejedor, M F; Goin, F J; Gelfo, J N; López, G; Bond, M; Carlini, A A; Scillato-Yané, G J; Woodburne, M O; Chornogubsky, L; Aragón, E; Reguero, M A; Czaplewski, N J; Vincon, S; Martin, G M; Ciancio, M R Tejedor, M F; Goin, F J; Gelfo, J N; López, G; Bond, M; Carlini, A A; Scillato-Yané, G J; Woodburne, M O; Chornogubsky, L; Aragón, E; Reguero, M A; Czaplewski, N J; Vincon, S; Martin, G M; Ciancio, M R (2009). New Early Eocene mammalian fauna from western Patagonia, Argentina. American Museum Novitates, (3638):1-43. Postprint available at: http://www.zora.uzh.ch Posted at the Zurich Open Repository and Archive, University of Zurich. http://www.zora.uzh.ch Originally published at: American Museum Novitates 2009, (3638):1-43. PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK, NY 10024 Number 3638, 43 pp., 10 figures, 1 table March 31, 2009 New Early Eocene Mammalian Fauna from Western Patagonia, Argentina MARCELO F.