Flying Primates Revisited: DNA Hybridization with Fractionated, GC-Enriched DNA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dating Placentalia: Morphological Clocks Fail to Close the Molecular-Fossil Gap
Puttick, M. N., Thomas, G. H., & Benton, M. J. (2016). Dating placentalia: Morphological clocks fail to close the molecular-fossil gap. Evolution, 70(4), 873-886. https://doi.org/10.1111/evo.12907 Publisher's PDF, also known as Version of record License (if available): CC BY Link to published version (if available): 10.1111/evo.12907 Link to publication record in Explore Bristol Research PDF-document © 2016 The Author(s). Evolution published by Wiley Periodicals, Inc. on behalf of The Society for the Study of Evolution. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ ORIGINAL ARTICLE doi:10.1111/evo.12907 Dating placentalia: Morphological clocks fail to close the molecular fossil gap Mark N. Puttick,1,2 Gavin H. Thomas,3 and Michael J. Benton1 1School of Earth Sciences, Life Sciences Building, Tyndall Avenue, University of Bristol, Bristol, BS8 1TQ, United Kingdom 2E-mail: [email protected] 3Department of Animal and Plant Sciences, Alfred Denny Building, University of Sheffield, Western Bank, Sheffield, S10 2TN, United Kingdom Received January 31, 2015 Accepted March 7, 2016 Dating the origin of Placentalia has been a contentious issue for biologists and paleontologists. -

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -
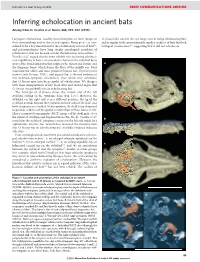
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

Mammalian Species 117
MAMMALIANSPECIES No. 117, pp. 14, 5 figs. Rhynchocyon chrysopygus. BY Galen B. Rathbun Published 8 June 1979 by the American Society of Mammalogists Rhynchocyon Peters, 1847 cyon chrysopygus apparently does not occur in the gallery forests of the Tana River, in the ground-water forest at Witu, or in the Rhynchocyon Peters, 1W7:36, type species Rhynchocyon cirnei dry bushlands between the Galana and Tana rivers. This ele- Peters by monotypy. phant-shrew's habitat is being cleared for exotic forest plantations Rhir~onaxThomas, 1918:370, type species Rh~nchoc~onchr~so- and agriculture all along the coast, resulting in a discontinuous pygus Gunther. and reduced distribution. It will re-occupy fallow agricultural land that is allowed to become overgrown with dense bush (Rathbun, CONTEXT AND CONTENT. Order Macroscelidea, Fam- ily Macroscelididae, Subfamily Rhynch~c~oninae.Corbet and unpublished data)' Hanks (1968) recognized three allopatric species of Rhynchocyon FOSSIL RECORD. As far as is known, the Macrosceli- (figure 1) for which they wrote the following key: didae have always been endemic to Africa (Patterson, 1%5). But- ler and Hopwood (1957) described Rhynchocyon clarki from the 1 Rump straw-colored, contrasting sharply with surround- early Miocene beds of Songhor, Kenya (near Lake Victoria). Ad- ing rufous pelage ----------.-------.-------R. chrysopygus ditional Miocene material from Rusinga Island, Kenya, has been Rump not straw-colored ---------.------.-------------------2 referred to this extinct form (Patterson, 1%5), which was smaller 2(1) Rump and posterior half of back with a pattern of dark than the extant species of Rhynchocyon. R. clarki contributes lines or spots on a yellowish-brown or rufous ground; significantly to the forest related fossil mammal fauna from Ru- top of head without a rufous tinge ._._._....._._R. -

Richard Dawkins, Bracketted V2.Wps
For all the many years that have passed away Matthew Lee Knowles 2010/11 (all the bracketed text extracted from ‘The Ancestor’s Tale’ by Richard Dawkins ) (and that is a myth too) (and in this context it always is man rather than woman) (although it baffles me why anybody regards this as an explanation for anything, given that the problem so swiftly regresses to the larger one of explaining the existence of the equally fine-tuned and improbable premeditator) (last long enough to make black holes, for instance) (though it often is) (the number of surviving species at the time of observation) (in the main a good book, so I shall not name and shame it) (a human species, probably ancestral to us) (boreal means northern) (and no less) (it is a intriguingly unfamiliar thought that there is always one such species) (including humans) (exactly in most cases, almost exactly in the rest) (it didn’t fossilise) (all but one of the other lineages went extinct) (quite a lot deeper into the past, and probably no longer in Africa) (most of) (or women) (or more) (petrified gum from trees) (as they tediously do) (or sixteen) (or hexadecimal) (see the Elephant Bird’s Tale) (especially microfossils) (see plate one) (tree rings) (carbon fourteen) (uranium-thorium-lead) (potassium-argon) (which I can sing) (or blossom, depending upon your taste) (unless, as has been recently suggested, their knotted strings were used for language as well as for counting) (the next copying ‘generation’) (it is the French hard c in comme) (American children call it ‘telephone’) -

103 the EVOLUTION of BATSI Leigh Van Valen Department Of
103 THE EVOLUTIONOF BATSI Leigh Van Valen Department of Biology University of Chicago 1103 East 57th Street Chicago, Illinois 60637 Received August 30, 1978; June 8, L979 Abstract: I devel-op the first explicitly Justified phylogeny of the known farnil-les of bats, usj.ng all available characters. This phylogeny permits the adaptive evolution of the order to be outlined. Two main clades emerge within the Microchiroptera and are cal-led new infraorders, Vespertilionia and Phyllostomatia. In each infraorder there is a series of grades of progressively stronger fi-ight, and there is a radiation of diet within the Phyllostomatia. Parallel evolution is extensive. Most grades stlll exist, presumably by a poorly understood partitioning of the resource space. The Megachiroptera nay have originated in the l-ate Ollgocene or early Miocene from surviving mernbers of the Eochiroptera (new suborder). The Kerivoul,ldae, Myzopodidae, Thyropteridae, and Furlpterldae are placed in the Natalidae, and the lcaronycterididae in the PaLaeochiropterygidae. The features of an ancestral bat are predicted. Bat origins are poorly known but may be found in Paleocene members of the Adapisoricidae. ,r** Bats constitute the second largest order of marnmalsand have a readily decipherable adaptive history, at least compared with the Rodentia and Insectivora. Nevertheless, there is no treatment of this adaptive hlstory nor even a general phyl-ogeny, which must form its foundation. I noticed this l-ack when revising a course on the paleobiology of mauunalsand undertook to remedy it. Although I sttll lack much familiarity with bats, the resul-t may be of more general interest. ORIGIN OF BATS One may hypothesize ttrat bats dld originate, but it is harder to go beyond this. -

The First Record of Anourosorex (Insectivora, Soricidae) from Western Myanmar, with Special Reference to Identification and Karyological Characters
Bull. Natl. Mus. Nat. Sci., Ser. A, 40(2), pp. 105–109, May 22, 2014 The First Record of Anourosorex (Insectivora, Soricidae) from Western Myanmar, with Special Reference to Identification and Karyological Characters Shin-ichiro Kawada1, Nozomi Kurihara1, Noriko Tominaga2 and Hideki Endo3 1 Department of Zoology, National Museum of Nature and Science, 4–1–1 Amakubo, Tsukuba, Ibaraki 305–0005, Japan E-mail: [email protected] 2 Adachi Study Center, The Open University of Japan, 5–13–5 Senju, Adachi-ku, Tokyo 120–0034, Japan 3 The University Museum, The University of Tokyo, 7–3–1 Hongo, Bunkyo, Tokyo 113–0033, Japan (Received 3 March 2014; accepted 26 March 2014) Abstract We collected six mole shrews in Tiddim Town of Chin State, western Myanmar. They were captured in a human-modified artificial environment, although mole shrews usually inhabit forests. External and skull measurements indicated that the species was Anourosorex assamensis, previously known only as an endemic species of the Assam region of India. This is the first record of this species from Myanmar. Karyological examination revealed the species was diploid with a fundamental autosomal number of 2n=50 and NFa=96. These numbers correspond to the Taiwanese species A. yamashinai, but several differences were apparent in chromosomal morphology and the position of secondary chromosomal constrictions. The karyological information suggests A. assamensis is a full species separate from A. squamipes in China. Key words : Mole shrew, Anourosorex assamensis, morphological identification, karyotype. karyotypes, and should therefore be considered Introduction separate species. After that, Hutterer (2005) Mole shrews, the genus Anourosorex Milne- reclassified all four Anourosorex as four distinct Edwards, 1872, are semifossorial shrews known species based on size differences. -

Morphological Diversity in Tenrecs (Afrosoricida, Tenrecidae)
Morphological diversity in tenrecs (Afrosoricida, Tenrecidae): comparing tenrec skull diversity to their closest relatives Sive Finlay and Natalie Cooper School of Natural Sciences, Trinity College Dublin, Dublin, Ireland Trinity Centre for Biodiversity Research, Trinity College Dublin, Dublin, Ireland ABSTRACT It is important to quantify patterns of morphological diversity to enhance our un- derstanding of variation in ecological and evolutionary traits. Here, we present a quantitative analysis of morphological diversity in a family of small mammals, the tenrecs (Afrosoricida, Tenrecidae). Tenrecs are often cited as an example of an ex- ceptionally morphologically diverse group. However, this assumption has not been tested quantitatively. We use geometric morphometric analyses of skull shape to test whether tenrecs are more morphologically diverse than their closest relatives, the golden moles (Afrosoricida, Chrysochloridae). Tenrecs occupy a wider range of ecological niches than golden moles so we predict that they will be more morpho- logically diverse. Contrary to our expectations, we find that tenrec skulls are only more morphologically diverse than golden moles when measured in lateral view. Furthermore, similarities among the species-rich Microgale tenrec genus appear to mask higher morphological diversity in the rest of the family. These results reveal new insights into the morphological diversity of tenrecs and highlight the impor- tance of using quantitative methods to test qualitative assumptions about patterns of morphological diversity. Submitted 29 January 2015 Subjects Evolutionary Studies, Zoology Accepted 13 April 2015 Keywords Golden moles, Geometric morphometrics, Disparity, Morphology Published 30 April 2015 Corresponding author Natalie Cooper, [email protected] INTRODUCTION Academic editor Analysing patterns of morphological diversity (the variation in physical form Foote, Laura Wilson 1997) has important implications for our understanding of ecological and evolutionary Additional Information and traits. -

Dating Placentalia: Morphological Clocks Fail to Close the Molecular Fossil Gap
View metadata, citation and similar papers at core.ac.uk brought to you by CORE ORIGINAL ARTICLE provided by White Rose Research Online doi:10.1111/evo.12907 Dating placentalia: Morphological clocks fail to close the molecular fossil gap Mark N. Puttick,1,2 Gavin H. Thomas,3 and Michael J. Benton1 1School of Earth Sciences, Life Sciences Building, Tyndall Avenue, University of Bristol, Bristol, BS8 1TQ, United Kingdom 2E-mail: [email protected] 3Department of Animal and Plant Sciences, Alfred Denny Building, University of Sheffield, Western Bank, Sheffield, S10 2TN, United Kingdom Received January 31, 2015 Accepted March 7, 2016 Dating the origin of Placentalia has been a contentious issue for biologists and paleontologists. Although it is likely that crown- group placentals originated in the Late Cretaceous, nearly all molecular clock estimates point to a deeper Cretaceous origin. An approach with the potential to reconcile this discrepancy could be the application of a morphological clock. This would permit the direct incorporation of fossil data in node dating, and would break long internal branches of the tree, so leading to improved estimates of node ages. Here, we use a large morphological dataset and the tip-calibration approach of MrBayes. We find that the estimated date for the origin of crown mammals is much older, 130–145 million years ago (Ma), than fossil and molecular clock data (80–90 Ma). Our results suggest that tip calibration may result in estimated dates that are more ancient than those obtained from other sources of data. This can be partially overcome by constraining the ages of internal nodes on the tree; however, when this was applied to our dataset, the estimated dates were still substantially more ancient than expected. -

Eutheria (Placental Mammals)
Eutheria (Placental Introductory article Mammals) Article Contents . Introduction J David Archibald, San Diego State University, San Diego, California, USA . Basic Design . Taxonomic and Ecological Diversity Eutheria includes one of three major clades of mammals, the extant members of which are . Fossil History and Distribution referred to as placentals. Phylogeny Introduction have supernumerary teeth (e.g. some whales, armadillos, Eutheria (or Placentalia) is the most taxonomically diverse etc.), in extant placentals the number of teeth is at most of three branches or clades of mammals, the other two three upper and lower incisors, one upper and lower being Metatheria (or Marsupialia) and Prototheria (or canine, four upper and lower premolars, and three upper Monotremata). When named by Gill in 1872, Eutheria and lower molars. Except for one fewer upper molar, a included both marsupials and placentals. It was Huxley in domestic dog retains this pattern. Compared to reptiles, 1880 that recognized Eutheria basically as used today to mammals have fewer skull bones through fusion and loss, include only placentals. McKenna and Bell in their although bones are variously emphasized in each of the Classification of Mammals, published in 1997, chose to three major mammalian taxa. use Placentalia rather than Eutheria to avoid the confusion Physiologically, mammals are all endotherms of varying of what taxa should be included in Eutheria. Others such as degrees of efficiency. They are also homeothermic with a Rougier have used Eutheria and Placentalia in the sense relatively high resting temperature. These characteristics used here. Placentalia includes all extant placentals and are also found in birds, but because of anatomical their most recent common ancestor. -

Molecular Phylogenetic Relationship Between and Within the Fruit
Journal of American Science, 2011;7(10) http://www.americanscience.org Molecular Phylogenetic Relationship Between and Within the Fruit Bat (Rousettus Aegyptiacus) and the Lesser Tailed Bat (Rhinopoma Hardwickei) Deduced From RAPD-PCR Analysis Ramadan A. M. Ali Department of Zoology, College for Women, Ain Shams University, Cairo, Egypt [email protected] Abstract: The RAPD-PCR in the present study was used to determine the genetic variation within and among two Egyptian bat species, Rousettus aegyptiacus and Rhinopoma hardwickei. The animals were captured from one locality at Giza governorate, Egypt. A total of 39 bands were amplified by the three primers OPAO2, OPAO8 and OPCO3 with an average 13 bands per primer at molecular weights ranged from 1409 to 107 bp. The polymorphic loci between both species were 34 with percentage 87.18 %. The numbers of monomorphic bands in Rousettus aegyptiacus aegyptiacus and Rhinopoma hardwickei arabium were 14 and 9 bands, respectively. The two species are sharing 5 (12.8 %) monomorphic bands. The similarity coefficients value between the two bat species was ranged from 0.353 to 0.500 with an average of 0.404 (40.4%). Dendrogram showed that, the two bats genotypes are separated from each other into two clusters and more variation among members of Rhinopoma hardwickei arabium was observed in comparison to those of Rousettus aegyptiacus aegyptiacus. It is concluded that, the similarity coefficient value between the two bat species indicates that, the two bat species may have the same origin but are not identical and separated into two clusters. [Ramadan A. M. Ali Molecular Phylogenetic Relationship Between and Within the Fruit Bat (Rousettus Aegyptiacus) and the Lesser Tailed Bat (Rhinopoma Hardwickei) Deduced From Rapd-Pcr Analysis] Journal of American Science 2011; 7(10): 678-687].(ISSN: 1545-1003). -

The Evolution of Echolocation in Bats: a Comparative Approach
The evolution of echolocation in bats: a comparative approach Alanna Collen A thesis submitted for the degree of Doctor of Philosophy from the Department of Genetics, Evolution and Environment, University College London. November 2012 Declaration Declaration I, Alanna Collen (née Maltby), confirm that the work presented in this thesis is my own. Where information has been derived from other sources, this is indicated in the thesis, and below: Chapter 1 This chapter is published in the Handbook of Mammalian Vocalisations (Maltby, Jones, & Jones) as a first authored book chapter with Gareth Jones and Kate Jones. Gareth Jones provided the research for the genetics section, and both Kate Jones and Gareth Jones providing comments and edits. Chapter 2 The raw echolocation call recordings in EchoBank were largely made and contributed by members of the ‘Echolocation Call Consortium’ (see full list in Chapter 2). The R code for the diversity maps was provided by Kamran Safi. Custom adjustments were made to the computer program SonoBat by developer Joe Szewczak, Humboldt State University, in order to select echolocation calls for measurement. Chapter 3 The supertree construction process was carried out using Perl scripts developed and provided by Olaf Bininda-Emonds, University of Oldenburg, and the supertree was run and dated by Olaf Bininda-Emonds. The source trees for the Pteropodidae were collected by Imperial College London MSc student Christina Ravinet. Chapter 4 Rob Freckleton, University of Sheffield, and Luke Harmon, University of Idaho, helped with R code implementation. 2 Declaration Chapter 5 Luke Harmon, University of Idaho, helped with R code implementation. Chapter 6 Joseph W.