1509831112.Full.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Of Vertebrate Fossils from the Middle Eocene Oil Shale of Messel, Germany: Implications for Their Taphonomy and Palaeoenvironment
Palaeogeography, Palaeoclimatology, Palaeoecology 416 (2014) 92–109 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Isotope compositions (C, O, Sr, Nd) of vertebrate fossils from the Middle Eocene oil shale of Messel, Germany: Implications for their taphonomy and palaeoenvironment Thomas Tütken ⁎ Steinmann-Institut für Geologie, Mineralogie und Paläontologie, Universität Bonn, Poppelsdorfer Schloss, 53115 Bonn, Germany article info abstract Article history: The Middle Eocene oil shale deposits of Messel are famous for their exceptionally well-preserved, articulated 47- Received 15 April 2014 Myr-old vertebrate fossils that often still display soft tissue preservation. The isotopic compositions (O, C, Sr, Nd) Received in revised form 30 July 2014 were analysed from skeletal remains of Messel's terrestrial and aquatic vertebrates to determine the condition of Accepted 5 August 2014 geochemical preservation. Authigenic phosphate minerals and siderite were also analysed to characterise the iso- Available online 17 August 2014 tope compositions of diagenetic phases. In Messel, diagenetic end member values of the volcanically-influenced 12 Keywords: and (due to methanogenesis) C-depleted anoxic bottom water of the meromictic Eocene maar lake are isoto- Strontium isotopes pically very distinct from in vivo bioapatite values of terrestrial vertebrates. This unique taphonomic setting al- Oxygen isotopes lows the assessment of the geochemical preservation of the vertebrate fossils. A combined multi-isotope Diagenesis approach demonstrates that enamel of fossil vertebrates from Messel is geochemically exceptionally well- Enamel preserved and still contains near-in vivo C, O, Sr and possibly even Nd isotope compositions while bone and den- Messel tine are diagenetically altered. -

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -
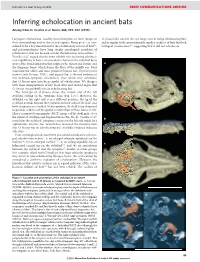
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

Arachnida: Araneae) from the Middle Eocene Messel Maar, Germany
Palaeoentomology 002 (6): 596–601 ISSN 2624-2826 (print edition) https://www.mapress.com/j/pe/ Short PALAEOENTOMOLOGY Copyright © 2019 Magnolia Press Communication ISSN 2624-2834 (online edition) PE https://doi.org/10.11646/palaeoentomology.2.6.10 http://zoobank.org/urn:lsid:zoobank.org:pub:E7F92F14-A680-4D30-8CF5-2B27C5AED0AB A new spider (Arachnida: Araneae) from the Middle Eocene Messel Maar, Germany PAUL A. SELDEN1, 2, * & torsten wappler3 1Department of Geology, University of Kansas, 1475 Jayhawk Boulevard, Lawrence, Kansas 66045, USA. 2Natural History Museum, Cromwell Road, London SW7 5BD, UK. 3Hessisches Landesmuseum Darmstadt, Friedensplatz 1, 64283 Darmstadt, Germany. *Corresponding author. E-mail: [email protected] The Fossil-Lagerstätte of Grube Messel, Germany, has Thomisidae and Salticidae (Schawaller & Ono, 1979; produced some of the most spectacular fossils of the Wunderlich, 1986). The Pliocene lake of Willershausen, Paleogene (Schaal & Ziegler, 1992; Gruber & Micklich, produced by solution of evaporites and subsequent collapse, 2007; Selden & Nudds, 2012; Schaal et al., 2018). However, has produced some remarkably preserved arthropod fossils few arachnids have been discovered or described from this (Briggs et al., 1998), including numerous spider families: World Heritage Site. An araneid spider was reported by Dysderidae, Lycosidae, Thomisidae and Salticidae (Straus, Wunderlich (1986). Wedmann (2018) reported that 160 1967; Schawaller, 1982). All of these localities are much spider specimens were known from Messel although, sadly, younger than Messel. few are well preserved. She figured the araneid mentioned by Wunderlich (1986) and a nicely preserved hersiliid (Wedmann, 2018: figs 7.8–7.9, respectively). Wedmann Material and methods (2018) mentioned six opilionids yet to be described, and figured one (Wedmann, 2018: fig. -

103 the EVOLUTION of BATSI Leigh Van Valen Department Of
103 THE EVOLUTIONOF BATSI Leigh Van Valen Department of Biology University of Chicago 1103 East 57th Street Chicago, Illinois 60637 Received August 30, 1978; June 8, L979 Abstract: I devel-op the first explicitly Justified phylogeny of the known farnil-les of bats, usj.ng all available characters. This phylogeny permits the adaptive evolution of the order to be outlined. Two main clades emerge within the Microchiroptera and are cal-led new infraorders, Vespertilionia and Phyllostomatia. In each infraorder there is a series of grades of progressively stronger fi-ight, and there is a radiation of diet within the Phyllostomatia. Parallel evolution is extensive. Most grades stlll exist, presumably by a poorly understood partitioning of the resource space. The Megachiroptera nay have originated in the l-ate Ollgocene or early Miocene from surviving mernbers of the Eochiroptera (new suborder). The Kerivoul,ldae, Myzopodidae, Thyropteridae, and Furlpterldae are placed in the Natalidae, and the lcaronycterididae in the PaLaeochiropterygidae. The features of an ancestral bat are predicted. Bat origins are poorly known but may be found in Paleocene members of the Adapisoricidae. ,r** Bats constitute the second largest order of marnmalsand have a readily decipherable adaptive history, at least compared with the Rodentia and Insectivora. Nevertheless, there is no treatment of this adaptive hlstory nor even a general phyl-ogeny, which must form its foundation. I noticed this l-ack when revising a course on the paleobiology of mauunalsand undertook to remedy it. Although I sttll lack much familiarity with bats, the resul-t may be of more general interest. ORIGIN OF BATS One may hypothesize ttrat bats dld originate, but it is harder to go beyond this. -

Micro CT Scanning of a New Specimen of the Zygodactylidae (Aves) from the Early Eocene of Messel, Germany
Master Thesis, Department of Geosciences Micro CT scanning of a new specimen of the Zygodactylidae (Aves) from the Early Eocene of Messel, Germany Christian Haugen Svendsen Micro CT scanning of a new specimen of the Zygodactylidae (Aves) from the early Eocene of Messel, Germany Christian Haugen Svendsen Master Thesis in Geosciences Discipline: Palaeontology Department of Geosciences and Natural History museum, Oslo Faculty of Mathematics and Natural Sciences University of Oslo September 2015 © Christian Haugen Svendsen, 2015 This work is published digitally through DUO – Digitale Utgivelser ved UiO http://www.duo.uio.no It is also catalogued in BIBSYS (http://www.bibsys.no/english) All rights reserved. No part of this publication may be reproduced or transmitted, in any form or by any means, without permission. Acknowledgement I want to thank and dedicate this work to my aunt Rita. Thanks for taking me to the Natural History Museum in Oslo when I was just a small kid. You helped me to get a general interest in the Evolution of life, and how complex we are, and this gave a 7 year old boy a dream. A dream to become a Palaeontologist! First and foremost I would like to thank my two excellent supervisors, Jørn Hurum and Gerald Mayr. Thanks for helping me whenever I had questions and for your suggestions on the thesis. Your patience, readiness and excellent guidance were crucial for this thesis. Special thanks to Gerald for meeting me in Frankfurt on my trip there, and showing me the collection at the Senckenberg museum. I want to thank the Senckenberg museum in Frankfurt for letting me examine the bird fossils from their records for my thesis. -

Fossil Ants (Hymenoptera: Formicidae): Ancient Diversity and the Rise of Modern Lineages
Myrmecological News 24 1-30 Vienna, March 2017 Fossil ants (Hymenoptera: Formicidae): ancient diversity and the rise of modern lineages Phillip BARDEN Abstract The ant fossil record is summarized with special reference to the earliest ants, first occurrences of modern lineages, and the utility of paleontological data in reconstructing evolutionary history. During the Cretaceous, from approximately 100 to 78 million years ago, only two species are definitively assignable to extant subfamilies – all putative crown group ants from this period are discussed. Among the earliest ants known are unexpectedly diverse and highly social stem- group lineages, however these stem ants do not persist into the Cenozoic. Following the Cretaceous-Paleogene boun- dary, all well preserved ants are assignable to crown Formicidae; the appearance of crown ants in the fossil record is summarized at the subfamilial and generic level. Generally, the taxonomic composition of Cenozoic ant fossil communi- ties mirrors Recent ecosystems with the "big four" subfamilies Dolichoderinae, Formicinae, Myrmicinae, and Ponerinae comprising most faunal abundance. As reviewed by other authors, ants increase in abundance dramatically from the Eocene through the Miocene. Proximate drivers relating to the "rise of the ants" are discussed, as the majority of this increase is due to a handful of highly dominant species. In addition, instances of congruence and conflict with molecular- based divergence estimates are noted, and distinct "ghost" lineages are interpreted. The ant fossil record is a valuable resource comparable to other groups with extensive fossil species: There are approximately as many described fossil ant species as there are fossil dinosaurs. The incorporation of paleontological data into neontological inquiries can only seek to improve the accuracy and scale of generated hypotheses. -

Testing for the Effects and Consequences of Mid Paleogene Climate Change on Insect Herbivory
Testing for the Effects and Consequences of Mid Paleogene Climate Change on Insect Herbivory Torsten Wappler1*, Conrad C. Labandeira2,3, Jes Rust1, Herbert Frankenha¨user4, Volker Wilde5 1 Steinmann Institute, University of Bonn, Bonn, Germany, 2 Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, District of Columbia, United States of America, 3 Department of Entomology and BEES Program, University of Maryland, College Park, Maryland, United States of America, 4 Mainz Natural History Museum/State Collection for Natural History of Rhineland-Palatine, Mainz, Germany, 5 Senckenberg Forschungsinstitut und Naturmuseum, Pala¨obotanik, Frankfurt am Main, Germany Abstract Background: The Eocene, a time of fluctuating environmental change and biome evolution, was generally driven by exceptionally warm temperatures. The Messel (47.8 Ma) and Eckfeld (44.3 Ma) deposits offer a rare opportunity to take a census of two, deep-time ecosystems occurring during a greenhouse system. An understanding of the long-term consequences of extreme warming and cooling events during this interval, particularly on angiosperms and insects that dominate terrestrial biodiversity, can provide insights into the biotic consequences of current global climatic warming. Methodology/Principal Findings: We compare insect-feeding damage within two middle Eocene fossil floras, Messel and Eckfeld, in Germany. From these small lake deposits, we studied 16,082 angiosperm leaves and scored each specimen for the presence or absence of 89 distinctive and diagnosable insect damage types (DTs), each of which was allocated to a major functional feeding group, including four varieties of external foliage feeding, piercing- and-sucking, leaf mining, galling, seed predation, and oviposition. Methods used for treatment of presence–absence data included general linear models and standard univariate, bivariate and multivariate statistical techniques. -

Messel Pit – Wikipedia Germany
03/08/2018 Messel pit - Wikipedia Coordinates: 49°55′03″N 8°45′24″E Messel pit The Messel Pit (German: Grube Messel) is a disused quarry near the Messel Pit Fossil Site village of Messel, (Landkreis Darmstadt-Dieburg, Hesse) about 35 km (22 mi) southeast of Frankfurt am Main, Germany. Bituminous shale UNESCO World Heritage site was mined there. Because of its abundance of fossils, it has significant geological and scientific importance. After almost becoming a landfill, strong local resistance eventually stopped these plans and the Messel Pit was declared a UNESCO World Heritage site on 9 December 1995. Significant scientific discoveries are still being made and the site has increasingly become a tourist site as well. Contents Location Darmstadt-Dieburg, History Hesse, Germany Depositional characteristics Criteria Natural: (viii) Volcanic gas releases Reference 720bis (http://whc.unesco. Fossils org/en/list/720bis) Mammals Inscription 1995 (19th Session) Birds Reptiles Extensions 2010 Fish Area 42 ha (4,500,000 sq ft) Insects Plants Buffer zone 22.5 ha (2,420,000 sq ft) Access Coordinates 49°55′03″N 8°45′24″E See also References External links History Brown coal and later oil shale was actively mined from 1859. The pit first became known for its wealth of fossils around 1900, but serious scientific excavation only started around the 1970s, when falling oil prices made the quarry uneconomical. Commercial oil shale mining ceased in 1971 and a cement factory built in the quarry failed the following year. The land was slotted for use as a landfill, but the plans came to nought and the Hessian state bought the site in 1991 to secure scientific access. -

To Scream Or to Listen? Prey Detection and Discrimination in Animal-Eating Bats
96 P.L. Jones et al. Fig. 4.1 Current phylogeny for bats (Jones and Teeling 2006 ). To the right of each family name, a Substrate Gleaner icon indicates that, in our opinion, this family is characterized by bat species that rely primarily on a gleaning strategy; all or most of which also take some prey by aerial hawk- ing. An Aerial Hawker icon indicates that the family consists of species most of which primarily use a hawking strategy but includes behaviorally fl exible species. Crasoenycteridae comprises a single behaviorally fl exible species. A Frugivore/Nectivore icon indicates a family comprised solely or partially of frugivorous and nectivorous species Each scenario, however, still suggests the same general story (Figure 4.1 ). That is, early laryngeal echolocating bats used powered fl ight, hunted animals, and took them in the air. The latter supposition is supported by the fact that most early fossil bats (~50 million years old) had wing designs suited for aerially hawking and not 4 Prey Detection and Discrimination in Animal-Eating Bats 97 like those of modern gleaners (Simmons and Geisler 1998 ; Safi et al. 2005 ). Therefore, we suppose that while something akin to gleaning may have characterized proto-bats and the very earliest of bats, this trait may have been subsequently lost, at least as a primary means of prey capture, as bats evolved more sophisticated laryngeal echolocation and longer, narrower wings, and then gleaning evolved inde- pendently again multiple times (Simmons and Geisler 1998 ) (Figure 4.1 ). However, while this idea is supported by fossil evidence (Simmons and Geisler 1998 ) and phylogenetic trait reconstructions (Safi et al. -

Integrated Fossil and Molecular Data Reconstruct Bat Echolocation
Integrated fossil and molecular data reconstruct bat echolocation Mark S. Springer†‡, Emma C. Teeling†§, Ole Madsen¶, Michael J. Stanhope§ʈ, and Wilfried W. de Jong¶** †Department of Biology, University of California, Riverside, CA 92521; §Queen’s University of Belfast, Biology and Biochemistry, 97 Lisburn Road, Belfast BT9 7BL, United Kingdom; ¶Department of Biochemistry, University of Nijmegen, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands; ʈBioinformatics, SmithKline Beecham Pharmaceuticals, 1250 South Collegeville Road, UP1345, Collegeville, PA 19426; and **Institute for Biodiversity and Ecosystem Dynamics, 1090 GT Amsterdam, The Netherlands Edited by David Pilbeam, Harvard University, Cambridge, MA, and approved March 26, 2001 (received for review November 21, 2000) Molecular and morphological data have important roles in illumi- tulates a sister-group relationship between flying lemurs and bats nating evolutionary history. DNA data often yield well resolved (6). Although some authors have questioned bat monophyly phylogenies for living taxa, but are generally unattainable for based on evidence from the penis and nervous system (7, 8), the fossils. A distinct advantage of morphology is that some types of bulk of morphological data supports bat monophyly (9). Among morphological data may be collected for extinct and extant taxa. living Chiroptera, morphological data provide strong support for Fossils provide a unique window on evolutionary history and may the reciprocal monophyly of megachiropterans (Old World fruit preserve combinations -

FOSSIL Project Updates Amateur Spotlight: by Bruce Macfadden & Eleanor Gardner Markmckinzie
News from the FOSSIL Project Vol. 2, Issue 4, December 2015 Inside this issue: Featured paleontologist: [email protected] myfossil.org @projectfossil The FossilProject Dena M. Smith FOSSIL Project Updates Amateur spotlight: by Bruce MacFadden & Eleanor Gardner MarkMcKinzie Recent Activities Club Corner: Rochester The FOSSIL Project collaborated with the Dallas Paleontological Society (DPS) and Academy of Science Fossil held a successful two-day mini conference on October 12 -13, 2015 prior to the Section Society of Vertebrate Paleontology (SVP) meeting. The mini conference included a tour of the paleontology collections at Southern Methodist University; informative Unlocking the Secrets of talks; a town-hall style forum with presentations by Dr. Scott Foss (Bureau of Land Fossil Color Management Senior Paleontologist), Linda McCall (North Carolina Fossil Club President), and Cynthia Crane (Aurora Fossil Museum Director); a well-attended and Lake Jacksboro Nautiloids inspiring keynote talk on bird origins by Dr. Paul Sereno of the University of Chicago; and a day-long field trip to fossil localities in the greater Dallas area. We thank Rocky Corkboard of Curiosities Manning, Lee Higginbotham, and the other members of the host/planning committee for an excellent time. After the mini conference, many of us then attended the SVP R.O.C.K.S. Update meeting in downtown Dallas, which included more than 1,000 professional and amateur paleontologists as well as students from the U.S. and around the world. Free Digitial Atlas App FOSSIL on Facebook Subscribe to our listserv www.myfossil.org/ subscribe/listserv/ Subscribe to newsletter www.myfossil.org/ subscribe/newsletter/ Jacksboro Field Site (DPS Trip) Mineral Wells (DPS Trip) continued from page 1 Immediately after the Dallas mini conference, Lisa Lundgren, a PhD student in Science Education at the University of Florida and the FOSSIL Project’s social media manager, presented research at the e-Learn international conference in Kona, Hawaii.