Of Vertebrate Fossils from the Middle Eocene Oil Shale of Messel, Germany: Implications for Their Taphonomy and Palaeoenvironment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Geoscience and a Lunar Base
" t N_iSA Conference Pubhcatmn 3070 " i J Geoscience and a Lunar Base A Comprehensive Plan for Lunar Explora, tion unclas HI/VI 02907_4 at ,unar | !' / | .... ._-.;} / [ | -- --_,,,_-_ |,, |, • • |,_nrrr|l , .l -- - -- - ....... = F _: .......... s_ dd]T_- ! JL --_ - - _ '- "_r: °-__.......... / _r NASA Conference Publication 3070 Geoscience and a Lunar Base A Comprehensive Plan for Lunar Exploration Edited by G. Jeffrey Taylor Institute of Meteoritics University of New Mexico Albuquerque, New Mexico Paul D. Spudis U.S. Geological Survey Branch of Astrogeology Flagstaff, Arizona Proceedings of a workshop sponsored by the National Aeronautics and Space Administration, Washington, D.C., and held at the Lunar and Planetary Institute Houston, Texas August 25-26, 1988 IW_A National Aeronautics and Space Administration Office of Management Scientific and Technical Information Division 1990 PREFACE This report was produced at the request of Dr. Michael B. Duke, Director of the Solar System Exploration Division of the NASA Johnson Space Center. At a meeting of the Lunar and Planetary Sample Team (LAPST), Dr. Duke (at the time also Science Director of the Office of Exploration, NASA Headquarters) suggested that future lunar geoscience activities had not been planned systematically and that geoscience goals for the lunar base program were not articulated well. LAPST is a panel that advises NASA on lunar sample allocations and also serves as an advocate for lunar science within the planetary science community. LAPST took it upon itself to organize some formal geoscience planning for a lunar base by creating a document that outlines the types of missions and activities that are needed to understand the Moon and its geologic history. -
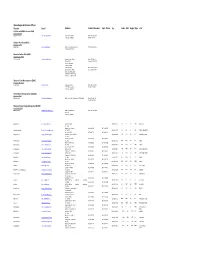
Alaska Regional Directors Offices Director Email Address Contact Numbers Supt
Alaska Regional Directors Offices Director Email Address Contact Numbers Supt. Phone Fax Code ABLI RegionType Unit U.S Fish and Wildlife Service (FWS) Alaska Region (FWS) HASKETT,GEOFFREY [email protected] 1011 East Tudor Road Phone: 907‐ 786‐3309 Anchorage, AK 99503 Fax: 907‐ 786‐3495 Naitonal Park Service(NPS) Alaska Region (NPS) MASICA,SUE [email protected] 240 West 5th Avenue,Suite 114 Phone:907‐644‐3510 Anchoorage,AK 99501 Bureau of Indian Affairs(BIA) Alaska Region (BIA) VIRDEN,EUGENE [email protected] Bureau of Indian Affairs Phone: 907‐586‐7177 PO Box 25520 Telefax: 907‐586‐7252 709 West 9th Street Juneau, AK 99802 Anchorage Agency Phone: 1‐800‐645‐8465 Bureau of Indian Affairs Telefax:907 271‐4477 3601 C Street Suite 1100 Anchorage, AK 99503‐5947 Telephone: 1‐800‐645‐8465 Bureau of Land Manangement (BLM) Alaska State Office (BLM) CRIBLEY,BUD [email protected] Alaska State Office Phone: 907‐271‐5960 222 W 7th Avenue #13 FAX: 907‐271‐3684 Anchorage, AK 99513 United States Geological Survey(USGS) Alaska Area (USGS) BARTELS,LESLIE lholland‐[email protected] 4210 University Dr., Anchorage, AK 99508‐4626 Phone:907‐786‐7055 Fax: 907‐ 786‐7040 Bureau of Ocean Energy Management(BOEM) Alaska Region (BOEM) KENDALL,JAMES [email protected] 3801 Centerpoint Drive Phone: 907‐ 334‐5208 Suite 500 Anchorage, AK 99503 Ralph Moore [email protected] c/o Katmai NP&P (907) 246‐2116 ANIA ANTI AKR NPRES ANIAKCHAK P.O. Box 7 King Salmon, AK 99613 (907) 246‐3305 (907) 246‐2120 Jeanette Pomrenke [email protected] P.O. -

The Sclerotic Ring: Evolutionary Trends in Squamates
The sclerotic ring: Evolutionary trends in squamates by Jade Atkins A Thesis Submitted to Saint Mary’s University, Halifax, Nova Scotia in Partial Fulfillment of the Requirements for the Degree of Master of Science in Applied Science July, 2014, Halifax Nova Scotia © Jade Atkins, 2014 Approved: Dr. Tamara Franz-Odendaal Supervisor Approved: Dr. Matthew Vickaryous External Examiner Approved: Dr. Tim Fedak Supervisory Committee Member Approved: Dr. Ron Russell Supervisory Committee Member Submitted: July 30, 2014 Dedication This thesis is dedicated to my family, friends, and mentors who helped me get to where I am today. Thank you. ! ii Table of Contents Title page ........................................................................................................................ i Dedication ...................................................................................................................... ii List of figures ................................................................................................................. v List of tables ................................................................................................................ vii Abstract .......................................................................................................................... x List of abbreviations and definitions ............................................................................ xi Acknowledgements .................................................................................................... -

Perissodactyla: Tapirus) Hints at Subtle Variations in Locomotor Ecology
JOURNAL OF MORPHOLOGY 277:1469–1485 (2016) A Three-Dimensional Morphometric Analysis of Upper Forelimb Morphology in the Enigmatic Tapir (Perissodactyla: Tapirus) Hints at Subtle Variations in Locomotor Ecology Jamie A. MacLaren1* and Sandra Nauwelaerts1,2 1Department of Biology, Universiteit Antwerpen, Building D, Campus Drie Eiken, Universiteitsplein, Wilrijk, Antwerp 2610, Belgium 2Centre for Research and Conservation, Koninklijke Maatschappij Voor Dierkunde (KMDA), Koningin Astridplein 26, Antwerp 2018, Belgium ABSTRACT Forelimb morphology is an indicator for order Perissodactyla (odd-toed ungulates). Modern terrestrial locomotor ecology. The limb morphology of the tapirs are widely accepted to belong to a single enigmatic tapir (Perissodactyla: Tapirus) has often been genus (Tapirus), containing four extant species compared to that of basal perissodactyls, despite the lack (Hulbert, 1973; Ruiz-Garcıa et al., 1985) and sev- of quantitative studies comparing forelimb variation in eral regional subspecies (Padilla and Dowler, 1965; modern tapirs. Here, we present a quantitative assess- ment of tapir upper forelimb osteology using three- Wilson and Reeder, 2005): the Baird’s tapir (T. dimensional geometric morphometrics to test whether bairdii), lowland tapir (T. terrestris), mountain the four modern tapir species are monomorphic in their tapir (T. pinchaque), and the Malayan tapir (T. forelimb skeleton. The shape of the upper forelimb bones indicus). Extant tapirs primarily inhabit tropical across four species (T. indicus; T. bairdii; T. terrestris; T. rainforest, with some populations also occupying pinchaque) was investigated. Bones were laser scanned wet grassland and chaparral biomes (Padilla and to capture surface morphology and 3D landmark analysis Dowler, 1965; Padilla et al., 1996). was used to quantify shape. -

Arachnida: Araneae) from the Middle Eocene Messel Maar, Germany
Palaeoentomology 002 (6): 596–601 ISSN 2624-2826 (print edition) https://www.mapress.com/j/pe/ Short PALAEOENTOMOLOGY Copyright © 2019 Magnolia Press Communication ISSN 2624-2834 (online edition) PE https://doi.org/10.11646/palaeoentomology.2.6.10 http://zoobank.org/urn:lsid:zoobank.org:pub:E7F92F14-A680-4D30-8CF5-2B27C5AED0AB A new spider (Arachnida: Araneae) from the Middle Eocene Messel Maar, Germany PAUL A. SELDEN1, 2, * & torsten wappler3 1Department of Geology, University of Kansas, 1475 Jayhawk Boulevard, Lawrence, Kansas 66045, USA. 2Natural History Museum, Cromwell Road, London SW7 5BD, UK. 3Hessisches Landesmuseum Darmstadt, Friedensplatz 1, 64283 Darmstadt, Germany. *Corresponding author. E-mail: [email protected] The Fossil-Lagerstätte of Grube Messel, Germany, has Thomisidae and Salticidae (Schawaller & Ono, 1979; produced some of the most spectacular fossils of the Wunderlich, 1986). The Pliocene lake of Willershausen, Paleogene (Schaal & Ziegler, 1992; Gruber & Micklich, produced by solution of evaporites and subsequent collapse, 2007; Selden & Nudds, 2012; Schaal et al., 2018). However, has produced some remarkably preserved arthropod fossils few arachnids have been discovered or described from this (Briggs et al., 1998), including numerous spider families: World Heritage Site. An araneid spider was reported by Dysderidae, Lycosidae, Thomisidae and Salticidae (Straus, Wunderlich (1986). Wedmann (2018) reported that 160 1967; Schawaller, 1982). All of these localities are much spider specimens were known from Messel although, sadly, younger than Messel. few are well preserved. She figured the araneid mentioned by Wunderlich (1986) and a nicely preserved hersiliid (Wedmann, 2018: figs 7.8–7.9, respectively). Wedmann Material and methods (2018) mentioned six opilionids yet to be described, and figured one (Wedmann, 2018: fig. -

Obituary 1967
OBITUARY INDEX 1967 You can search by clicking on the binoculars on the adobe toolbar or by Pressing Shift-Control-F Request Form LAST NAME FIRST NAME DATE PAGE # Abbate Barbara F. 6/6/1967 p.1 Abbott William W. 9/22/1967 p.30 Abel Dean 11/27/1967 p.24 Abel Francis E. 9/25/1967 p.24 Abel Francis E. 9/27/1967 p.10 Abel Frederick B. 11/11/1967 p.26 Abel John Hawk 12/7/1967 p.42 Abel John Hawk 12/11/1967 p.26 Abel William E. 7/10/1967 p.28 Abel William E. 7/12/1967 p.12 Aber George W. 3/14/1967 p.24 Abert Esther F. 7/26/1967 p.14 Abrams Laura R. Hoffman 8/14/1967 p.28 Abrams Laura R. Hoffman 8/17/1967 p.28 Abrams Pearl E. 10/19/1967 p.28 Abrams Pearl E. 10/20/1967 p.10 Achenbach Helen S. 11/6/1967 p.36 Achenbach Helen S. 11/8/1967 p.15 Ackerman Calvin 5/22/1967 p.34 Ackerman Calvin 5/25/1967 p.38 Ackerman Fritz 11/27/1967 p.24 Ackerman Harold 10/27/1967 p.26 Ackerman Harold 10/28/1967 p.26 Ackerman Hattie Mann 4/14/1967 p.21 Ackerman Hattie Mann 4/19/1967 p.14 Adamczyk Bennie 5/3/1967 p.14 Adamo Margaret M. 3/15/1967 p.15 Adamo Margaret M. 3/20/1967 p.28 Adams Anna 6/17/1967 p.22 Adams Anna 6/20/1967 p.17 Adams Harriet C. -

Critical Comments on the Genus Propachynolophus Lemoine, 1891 (Mammalia, Perissodactyla, Equoidea)
ARTICLE Critical comments on the genus Propachynolophus Lemoine, 1891 (Mammalia, Perissodactyla, Equoidea) JEAN A. REMY Institut des Sciences de l’Evolution, UMR 5554 CNRS, IRD, EPHE, Université de Montpellier, Place Eugène Bataillon, 34095 Montpellier cedex 5, France E-mail: [email protected] Abstract: The validity of Propachynolophus Lemoine, 1891, supposedly an intermediate between Hyracotherium Owen, 1841 and Pachynolophus Pomel, 1847, has been questioned for a long time. A detailed analysis of features on which this genus is based further supported by a formal cladistic analysis demonstrates that Propachynolophus is not a valid taxon. The type species, “Propachynolophus gaudryi Lemoine, 1891” shall be assigned to Propalaeotherium Gervais, 1849, under the new combination Propalaeotherium gaudryi (Lemoine, 1891). “Pachynolophus maldani Lemoine, 1878”, later assigned to Propachynolophus, typifies the new genus Orolophus, under the binomen Orolophus maldani (Lemoine, 1878). The other referred species, “Propachynolophus levei Hooker, 1994” and “P. remyi Checa-Soler, 1997” are poorly documented, and both species shall be provisionally referred to as “Hyracotherium” levei (Hooker, 1994) and “Hyracotherium” remyi (Checa-Soler, 1997), pending new discoveries. Keywords: Eocene, tooth morphology, Pachynolophus, Propalaeotherium, Eurohippus Submitted 8 December 2016, Accepted 3 August 2017 Published Online 27 September 2017, doi: 1018563/pv.41.1.e3 © Copyright Jean Remy September 2017 INTRODUCTION Propachynolophus gaudryi was “based upon miscellaneous isolated teeth and jaw fragments constituting a part of the fossil vertebrate sample collected in the vicinity of Epernay (Marne)” By their diversity and abundance, perissodactyls have played (Savage et al., 1965: 16), a sample known as the “Ageian a crucial role in the European Eocene mammalian faunas. -

The Making of Calibration Sausage Exemplified by Recalibrating the Transcriptomic Timetree of Jawed Vertebrates
bioRxiv preprint doi: https://doi.org/10.1101/2019.12.19.882829; this version posted January 5, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. The making of calibration sausage exemplified by recalibrating the transcriptomic timetree of jawed vertebrates 1 David Marjanović 2 Department of Evolutionary Morphology, Science Programme “Evolution and Geoprocesses”, 3 Museum für Naturkunde – Leibniz Institute for Evolutionary and Biodiversity Research, Berlin, 4 Germany 5 ORCID: 0000-0001-9720-7726 6 Correspondence: 7 David Marjanović 8 [email protected] 9 Keywords: timetree, calibration, divergence date, Gnathostomata, Vertebrata 10 Abstract 11 Molecular divergence dating has the potential to overcome the incompleteness of the fossil record in 12 inferring when cladogenetic events (splits, divergences) happened, but needs to be calibrated by the 13 fossil record. Ideally but unrealistically, this would require practitioners to be specialists in molecular 14 evolution, in the phylogeny and the fossil record of all sampled taxa, and in the chronostratigraphy of 15 the sites the fossils were found in. Paleontologists have therefore tried to help by publishing 16 compendia of recommended calibrations, and molecular biologists unfamiliar with the fossil record 17 have made heavy use of such works (in addition to using scattered primary sources -

Final Copy 2019 10 01 Herrera
This electronic thesis or dissertation has been downloaded from Explore Bristol Research, http://research-information.bristol.ac.uk Author: Herrera Flores, Jorge Alfredo A Title: The macroevolution and macroecology of Mesozoic lepidosaurs General rights Access to the thesis is subject to the Creative Commons Attribution - NonCommercial-No Derivatives 4.0 International Public License. A copy of this may be found at https://creativecommons.org/licenses/by-nc-nd/4.0/legalcode This license sets out your rights and the restrictions that apply to your access to the thesis so it is important you read this before proceeding. Take down policy Some pages of this thesis may have been removed for copyright restrictions prior to having it been deposited in Explore Bristol Research. However, if you have discovered material within the thesis that you consider to be unlawful e.g. breaches of copyright (either yours or that of a third party) or any other law, including but not limited to those relating to patent, trademark, confidentiality, data protection, obscenity, defamation, libel, then please contact [email protected] and include the following information in your message: •Your contact details •Bibliographic details for the item, including a URL •An outline nature of the complaint Your claim will be investigated and, where appropriate, the item in question will be removed from public view as soon as possible. This electronic thesis or dissertation has been downloaded from Explore Bristol Research, http://research-information.bristol.ac.uk Author: Herrera Flores, Jorge Alfredo A Title: The macroevolution and macroecology of Mesozoic lepidosaurs General rights Access to the thesis is subject to the Creative Commons Attribution - NonCommercial-No Derivatives 4.0 International Public License. -

Appendix I Lunar and Martian Nomenclature
APPENDIX I LUNAR AND MARTIAN NOMENCLATURE LUNAR AND MARTIAN NOMENCLATURE A large number of names of craters and other features on the Moon and Mars, were accepted by the IAU General Assemblies X (Moscow, 1958), XI (Berkeley, 1961), XII (Hamburg, 1964), XIV (Brighton, 1970), and XV (Sydney, 1973). The names were suggested by the appropriate IAU Commissions (16 and 17). In particular the Lunar names accepted at the XIVth and XVth General Assemblies were recommended by the 'Working Group on Lunar Nomenclature' under the Chairmanship of Dr D. H. Menzel. The Martian names were suggested by the 'Working Group on Martian Nomenclature' under the Chairmanship of Dr G. de Vaucouleurs. At the XVth General Assembly a new 'Working Group on Planetary System Nomenclature' was formed (Chairman: Dr P. M. Millman) comprising various Task Groups, one for each particular subject. For further references see: [AU Trans. X, 259-263, 1960; XIB, 236-238, 1962; Xlffi, 203-204, 1966; xnffi, 99-105, 1968; XIVB, 63, 129, 139, 1971; Space Sci. Rev. 12, 136-186, 1971. Because at the recent General Assemblies some small changes, or corrections, were made, the complete list of Lunar and Martian Topographic Features is published here. Table 1 Lunar Craters Abbe 58S,174E Balboa 19N,83W Abbot 6N,55E Baldet 54S, 151W Abel 34S,85E Balmer 20S,70E Abul Wafa 2N,ll7E Banachiewicz 5N,80E Adams 32S,69E Banting 26N,16E Aitken 17S,173E Barbier 248, 158E AI-Biruni 18N,93E Barnard 30S,86E Alden 24S, lllE Barringer 29S,151W Aldrin I.4N,22.1E Bartels 24N,90W Alekhin 68S,131W Becquerei -

Lick Observatory Records: Photographs UA.036.Ser.07
http://oac.cdlib.org/findaid/ark:/13030/c81z4932 Online items available Lick Observatory Records: Photographs UA.036.Ser.07 Kate Dundon, Alix Norton, Maureen Carey, Christine Turk, Alex Moore University of California, Santa Cruz 2016 1156 High Street Santa Cruz 95064 [email protected] URL: http://guides.library.ucsc.edu/speccoll Lick Observatory Records: UA.036.Ser.07 1 Photographs UA.036.Ser.07 Contributing Institution: University of California, Santa Cruz Title: Lick Observatory Records: Photographs Creator: Lick Observatory Identifier/Call Number: UA.036.Ser.07 Physical Description: 101.62 Linear Feet127 boxes Date (inclusive): circa 1870-2002 Language of Material: English . https://n2t.net/ark:/38305/f19c6wg4 Conditions Governing Access Collection is open for research. Conditions Governing Use Property rights for this collection reside with the University of California. Literary rights, including copyright, are retained by the creators and their heirs. The publication or use of any work protected by copyright beyond that allowed by fair use for research or educational purposes requires written permission from the copyright owner. Responsibility for obtaining permissions, and for any use rests exclusively with the user. Preferred Citation Lick Observatory Records: Photographs. UA36 Ser.7. Special Collections and Archives, University Library, University of California, Santa Cruz. Alternative Format Available Images from this collection are available through UCSC Library Digital Collections. Historical note These photographs were produced or collected by Lick observatory staff and faculty, as well as UCSC Library personnel. Many of the early photographs of the major instruments and Observatory buildings were taken by Henry E. Matthews, who served as secretary to the Lick Trust during the planning and construction of the Observatory.