Evolution of Body Mass in Bats: Insights from a Large Supermatrix Phylogeny
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Neoichnology of Bats: Morphological, Ecological, and Phylogenetic Influences on Terrestrial Behavior and Trackmaking Ability Within the Chiroptera
NEOICHNOLOGY OF BATS: MORPHOLOGICAL, ECOLOGICAL, AND PHYLOGENETIC INFLUENCES ON TERRESTRIAL BEHAVIOR AND TRACKMAKING ABILITY WITHIN THE CHIROPTERA BY MATTHEW FRAZER JONES Submitted to the graduate degree program in Geology and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Master of Science. Advisory Committee: ______________________________ Chairperson Stephen T. Hasiotis ______________________________ Co-chair David A. Burnham ______________________________ Robert M. Timm Date Defended: April 8, 2016 The Thesis Committee for MATTHEW FRAZER JONES certifies that this is the approved version of the following thesis: NEOICHNOLOGY OF BATS: MORPHOLOGICAL, ECOLOGICAL, AND PHYLOGENETIC INFLUENCES ON TERRESTRIAL BEHAVIOR AND TRACKMAKING ABILITY WITHIN THE CHIROPTERA ______________________________ Chairperson: Stephen T. Hasiotis ______________________________ Co-chairperson: David A. Burnham Date Approved: April 8, 2016 ii ABSTRACT Among living mammals, bats (Chiroptera) are second only to rodents in total number of species with over 1100 currently known. Extant bat species occupy many trophic niches and feeding habits, including frugivores (fruit eaters), insectivores (insect eaters), nectarivores (nectar and pollen-eaters), carnivores (predators of small terrestrial vertebrates), piscivores (fish eaters), sanguinivores (blood eaters), and omnivores (eat animals and plant material). Modern bats also demonstrate a wide range of terrestrial abilities while feeding, including: (1) those that primarily feed at or near ground level, such as the common vampire bat (Desmodus rotundus) and the New Zealand short-tailed bat (Mystacina tuberculata); (2) those rarely observed to feed from or otherwise spend time on the ground; and (3) many intermediate forms that demonstrate terrestrial competency without an obvious ecological basis. The variation in chiropteran terrestrial ability has been hypothesized to be constrained by the morphology of the pelvis and hindlimbs into what are termed types 1, 2, and 3 bats. -

Timeline of the Evolutionary History of Life
Timeline of the evolutionary history of life This timeline of the evolutionary history of life represents the current scientific theory Life timeline Ice Ages outlining the major events during the 0 — Primates Quater nary Flowers ←Earliest apes development of life on planet Earth. In P Birds h Mammals – Plants Dinosaurs biology, evolution is any change across Karo o a n ← Andean Tetrapoda successive generations in the heritable -50 0 — e Arthropods Molluscs r ←Cambrian explosion characteristics of biological populations. o ← Cryoge nian Ediacara biota – z ← Evolutionary processes give rise to diversity o Earliest animals ←Earliest plants at every level of biological organization, i Multicellular -1000 — c from kingdoms to species, and individual life ←Sexual reproduction organisms and molecules, such as DNA and – P proteins. The similarities between all present r -1500 — o day organisms indicate the presence of a t – e common ancestor from which all known r Eukaryotes o species, living and extinct, have diverged -2000 — z o through the process of evolution. More than i Huron ian – c 99 percent of all species, amounting to over ←Oxygen crisis [1] five billion species, that ever lived on -2500 — ←Atmospheric oxygen Earth are estimated to be extinct.[2][3] Estimates on the number of Earth's current – Photosynthesis Pong ola species range from 10 million to 14 -3000 — A million,[4] of which about 1.2 million have r c been documented and over 86 percent have – h [5] e not yet been described. However, a May a -3500 — n ←Earliest oxygen 2016 -
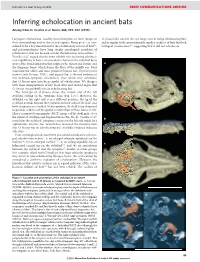
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

BIO 313 ANIMAL ECOLOGY Corrected
NATIONAL OPEN UNIVERSITY OF NIGERIA SCHOOL OF SCIENCE AND TECHNOLOGY COURSE CODE: BIO 314 COURSE TITLE: ANIMAL ECOLOGY 1 BIO 314: ANIMAL ECOLOGY Team Writers: Dr O.A. Olajuyigbe Department of Biology Adeyemi Colledge of Education, P.M.B. 520, Ondo, Ondo State Nigeria. Miss F.C. Olakolu Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. Mrs H.O. Omogoriola Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. EDITOR: Mrs Ajetomobi School of Agricultural Sciences Lagos State Polytechnic Ikorodu, Lagos 2 BIO 313 COURSE GUIDE Introduction Animal Ecology (313) is a first semester course. It is a two credit unit elective course which all students offering Bachelor of Science (BSc) in Biology can take. Animal ecology is an important area of study for scientists. It is the study of animals and how they related to each other as well as their environment. It can also be defined as the scientific study of interactions that determine the distribution and abundance of organisms. Since this is a course in animal ecology, we will focus on animals, which we will define fairly generally as organisms that can move around during some stages of their life and that must feed on other organisms or their products. There are various forms of animal ecology. This includes: • Behavioral ecology, the study of the behavior of the animals with relation to their environment and others • Population ecology, the study of the effects on the population of these animals • Marine ecology is the scientific study of marine-life habitat, populations, and interactions among organisms and the surrounding environment including their abiotic (non-living physical and chemical factors that affect the ability of organisms to survive and reproduce) and biotic factors (living things or the materials that directly or indirectly affect an organism in its environment). -

Chiroptera: Pteropodidae)
Chapter 6 Phylogenetic Relationships of Harpyionycterine Megabats (Chiroptera: Pteropodidae) NORBERTO P. GIANNINI1,2, FRANCISCA CUNHA ALMEIDA1,3, AND NANCY B. SIMMONS1 ABSTRACT After almost 70 years of stability following publication of Andersen’s (1912) monograph on the group, the systematics of megachiropteran bats (Chiroptera: Pteropodidae) was thrown into flux with the advent of molecular phylogenetics in the 1980s—a state where it has remained ever since. One particularly problematic group has been the Austromalayan Harpyionycterinae, currently thought to include Dobsonia and Harpyionycteris, and probably also Aproteles.Inthis contribution we revisit the systematics of harpyionycterines. We examine historical hypotheses of relationships including the suggestion by O. Thomas (1896) that the rousettine Boneia bidens may be related to Harpyionycteris, and report the results of a series of phylogenetic analyses based on new as well as previously published sequence data from the genes RAG1, RAG2, vWF, c-mos, cytb, 12S, tVal, 16S,andND2. Despite a striking lack of morphological synapomorphies, results of our combined analyses indicate that Boneia groups with Aproteles, Dobsonia, and Harpyionycteris in a well-supported, expanded Harpyionycterinae. While monophyly of this group is well supported, topological changes within this clade across analyses of different data partitions indicate conflicting phylogenetic signals in the mitochondrial partition. The position of the harpyionycterine clade within the megachiropteran tree remains somewhat uncertain. Nevertheless, biogeographic patterns (vicariance-dispersal events) within Harpyionycterinae appear clear and can be directly linked to major biogeographic boundaries of the Austromalayan region. The new phylogeny of Harpionycterinae also provides a new framework for interpreting aspects of dental evolution in pteropodids (e.g., reduction in the incisor dentition) and allows prediction of roosting habits for Harpyionycteris, whose habits are unknown. -

Figs1 ML Tree.Pdf
100 Megaderma lyra Rhinopoma hardwickei 71 100 Rhinolophus creaghi 100 Rhinolophus ferrumequinum 100 Hipposideros armiger Hipposideros commersoni 99 Megaerops ecaudatus 85 Megaerops niphanae 100 Megaerops kusnotoi 100 Cynopterus sphinx 98 Cynopterus horsfieldii 69 Cynopterus brachyotis 94 50 Ptenochirus minor 86 Ptenochirus wetmorei Ptenochirus jagori Dyacopterus spadiceus 99 Sphaerias blanfordi 99 97 Balionycteris maculata 100 Aethalops alecto 99 Aethalops aequalis Thoopterus nigrescens 97 Alionycteris paucidentata 33 99 Haplonycteris fischeri 29 Otopteropus cartilagonodus Latidens salimalii 43 88 Penthetor lucasi Chironax melanocephalus 90 Syconycteris australis 100 Macroglossus minimus 34 Macroglossus sobrinus 92 Boneia bidens 100 Harpyionycteris whiteheadi 69 Harpyionycteris celebensis Aproteles bulmerae 51 Dobsonia minor 100 100 80 Dobsonia inermis Dobsonia praedatrix 99 96 14 Dobsonia viridis Dobsonia peronii 47 Dobsonia pannietensis 56 Dobsonia moluccensis 29 Dobsonia anderseni 100 Scotonycteris zenkeri 100 Casinycteris ophiodon 87 Casinycteris campomaanensis Casinycteris argynnis 99 100 Eonycteris spelaea 100 Eonycteris major Eonycteris robusta 100 100 Rousettus amplexicaudatus 94 Rousettus spinalatus 99 Rousettus leschenaultii 100 Rousettus aegyptiacus 77 Rousettus madagascariensis 87 Rousettus obliviosus Stenonycteris lanosus 100 Megaloglossus woermanni 100 91 Megaloglossus azagnyi 22 Myonycteris angolensis 100 87 Myonycteris torquata 61 Myonycteris brachycephala 33 41 Myonycteris leptodon Myonycteris relicta 68 Plerotes anchietae -

Taxonomy and Affinities of African Cenozoic Metatherians
Spanish Journal of Palaeontology 36 (2), 2021 https://doi.org/10.7203/sjp.36.2.20974 Sociedad Española de Paleontología ISSN 2255-0550 / eISSN 2660-9568 OPEN ACCESS RESEARCH PAPER Taxonomy and affi nities of african cenozoic metatherians Taxonomía y afi nidades de los metaterios cenozoicos africanos Vicente D. CRESPO & Francisco J. GOIN Abstract: The record of extinct African metatherians (Mammalia, Theria) is scanty, restricted Received: 20 January 2021 in time (Eocene–Miocene), and its taxonomy is still subject of debate. A review of all African Accepted: 24 May 2021 metatherians, or alleged metatherians, known up to now, led us to the recognition of only Published online: XXX three taxa referable to this group: (1) Kasserinotherium tunisiense (Peradectoidea?), from the early Eocene of Tunisia; (2) Peratherium africanum (Herpetotheriidae), from the early Oligocene of Egypt and Oman, and (3) an indeterminate Herpetotheriidae? from the early Corresponding author: Miocene of Uganda. Herpetotheriids probably reached Afro-Arabia from Europe in one Vicente D. Crespo or more dispersal waves since the early Oligocene. Kasserinotherium, on the contrary, [email protected] suggests an earlier (Paleocene) arrival from South America, judging from its alleged affi nities with South American and Australian taxa. Such a migration event (probably, Keywords: through a fi lter corridor such as the Rio Grande Rise-Walvis Ridge system in the South Mammalia Atlantic) may also explain the enigmatic presence of polydolopimorphian metatherians in Metatheria the Cenozoic of central Anatolia (Turkey). A more radical hypothesis is that all European (Eurasian?) Marsupialiformes have an ultimate origin in South America, from where they Africa dispersed via Africa by the Paleocene–earliest Eocene. -

Proquest Dissertations
Universite de Montreal Chimeres, donnees manquantes et congruence: validation de differentes methodes par simulations et application a la phylogenie des mammiferes par Veronique Campbell Departement de sciences biologiques Faculte des arts et sciences These presentee a la Faculte des etudes superieures en vue de I'obtention du grade de Philosophiae doctor (Ph. D.) en sciences biologiques Aout, 2009 © Veronique Campbell, 200 Library and Archives Bibliotheque et 1*1 Canada Archives Canada Published Heritage Direction du Branch Patrimoine de I'edition 395 Wellington Street 395, rue Wellington Ottawa ON K1A 0N4 OttawaONK1A0N4 Canada Canada Your file Votre reference ISBN: 978-0-494-60679-7 Our file Notre reference ISBN: 978-0-494-60679-7 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library and permettant a la Bibliotheque et Archives Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par I'lnternet, prefer, telecommunication or on the Internet, distribuer et vendre des theses partout dans le loan, distribute and sell theses monde, a des fins commerciales ou autres, sur worldwide, for commercial or non support microforme, papier, electronique et/ou commercial purposes, in microform, autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve la propriete du droit d'auteur ownership and moral rights in this et des droits moraux qui protege cette these. Ni thesis. Neither the thesis nor la these ni des extraits substantiels de celle-ci substantial extracts from it may be ne doivent etre imprimes ou autrement printed or otherwise reproduced reproduits sans son autorisation. -

Bat Count 2003
BAT COUNT 2003 Working to promote the long term, sustainable conservation of globally threatened flying foxes in the Philippines, by developing baseline population information, increasing public awareness, and training students and protected area managers in field monitoring techniques. 1 A Terminal Report Submitted by Tammy Mildenstein1, Apolinario B. Cariño2, and Samuel Stier1 1Fish and Wildlife Biology, University of Montana, USA 2Silliman University and Mt. Talinis – Twin Lakes Federation of People’s Organizations, Diputado Extension, Sibulan, Negros Oriental, Philippines Photo by: Juan Pablo Moreiras 2 EXECUTIVE SUMMARY Large flying foxes in insular Southeast Asia are the most threatened of the Old World fruit bats due to deforestation, unregulated hunting, and little conservation commitment from local governments. Despite the fact they are globally endangered and play essential ecological roles in forest regeneration as seed dispersers and pollinators, there have been only a few studies on these bats that provide information useful to their conservation management. Our project aims to promote the conservation of large flying foxes in the Philippines by providing protected area managers with the training and the baseline information necessary to design and implement a long-term management plan for flying foxes. We focused our efforts on the globally endangered Philippine endemics, Acerodon jubatus and Acerodon leucotis, and the bats that commonly roost with them, Pteropus hypomelanus, P. vampyrus lanensis, and P. pumilus which are thought to be declining in the Philippines. Local participation is an integral part of our project. We conducted the first national training workshop on flying fox population counts and conservation at the Subic Bay area. -

103 the EVOLUTION of BATSI Leigh Van Valen Department Of
103 THE EVOLUTIONOF BATSI Leigh Van Valen Department of Biology University of Chicago 1103 East 57th Street Chicago, Illinois 60637 Received August 30, 1978; June 8, L979 Abstract: I devel-op the first explicitly Justified phylogeny of the known farnil-les of bats, usj.ng all available characters. This phylogeny permits the adaptive evolution of the order to be outlined. Two main clades emerge within the Microchiroptera and are cal-led new infraorders, Vespertilionia and Phyllostomatia. In each infraorder there is a series of grades of progressively stronger fi-ight, and there is a radiation of diet within the Phyllostomatia. Parallel evolution is extensive. Most grades stlll exist, presumably by a poorly understood partitioning of the resource space. The Megachiroptera nay have originated in the l-ate Ollgocene or early Miocene from surviving mernbers of the Eochiroptera (new suborder). The Kerivoul,ldae, Myzopodidae, Thyropteridae, and Furlpterldae are placed in the Natalidae, and the lcaronycterididae in the PaLaeochiropterygidae. The features of an ancestral bat are predicted. Bat origins are poorly known but may be found in Paleocene members of the Adapisoricidae. ,r** Bats constitute the second largest order of marnmalsand have a readily decipherable adaptive history, at least compared with the Rodentia and Insectivora. Nevertheless, there is no treatment of this adaptive hlstory nor even a general phyl-ogeny, which must form its foundation. I noticed this l-ack when revising a course on the paleobiology of mauunalsand undertook to remedy it. Although I sttll lack much familiarity with bats, the resul-t may be of more general interest. ORIGIN OF BATS One may hypothesize ttrat bats dld originate, but it is harder to go beyond this. -

The Philippine Flying Foxes, Acerodon Jubatus and Pteropus Vampyrus Lanensis
Journal of Mammalogy, 86(4):719- 728, 2005 DIETARY HABITS OF THE WORLD’S LARGEST BATS: THE PHILIPPINE FLYING FOXES, ACERODON JUBATUS AND PTEROPUS VAMPYRUS LANENSIS Sam C. Stier* and Tammy L. M ildenstein College of Forestry and Conservation, University of Montana, Missoula, MT 59802, USA The endemic and endangered golden- crowned flying fox (Acerodon jubatus) coroosts with the much more common and widespread giant Philippine fmit bat (Pteropus vampyrus ianensis) in lowland dipterocarp forests throughout the Philippine Islands. The number of these mixed roost- colonies and the populations of flying foxes in them have declined dramatically in the last century. We used fecal analysis, interviews of bat hunters, and personal observations to describe the dietary habits of both bat species at one of the largest mixed roosts remaining, near Subic Bay, west- central Luzon. Dietary items were deemed “important” if used consistently on a seasonal basis or throughout the year, ubiquitously throughout the population, and if they were of clear nutritional value. Of the 771 droppings examined over a 2.5 -year period (1998-2000), seeds from Ficus were predominant in the droppings of both species and met these criteria, particularly hemiepiphytic species (41% of droppings of A. jubatus) and Ficus variegata (34% of droppings of P. v. ianensis and 22% of droppings of A. jubatus). Information from bat hunter interviews expanded our knowledge of the dietary habits of both bat species, and corroborated the fecal analyses and personal observations. Results from this study suggest that A. jubatus is a forest obligate, foraging on fruits and leaves from plant species restricted to lowland, mature natural forests, particularly using a small subset of hemiepiphytic and other Ficus species throughout the year.