Evidence for Repeated Independent Evolution of Migration in the Largest Family of Bats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Neoichnology of Bats: Morphological, Ecological, and Phylogenetic Influences on Terrestrial Behavior and Trackmaking Ability Within the Chiroptera
NEOICHNOLOGY OF BATS: MORPHOLOGICAL, ECOLOGICAL, AND PHYLOGENETIC INFLUENCES ON TERRESTRIAL BEHAVIOR AND TRACKMAKING ABILITY WITHIN THE CHIROPTERA BY MATTHEW FRAZER JONES Submitted to the graduate degree program in Geology and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Master of Science. Advisory Committee: ______________________________ Chairperson Stephen T. Hasiotis ______________________________ Co-chair David A. Burnham ______________________________ Robert M. Timm Date Defended: April 8, 2016 The Thesis Committee for MATTHEW FRAZER JONES certifies that this is the approved version of the following thesis: NEOICHNOLOGY OF BATS: MORPHOLOGICAL, ECOLOGICAL, AND PHYLOGENETIC INFLUENCES ON TERRESTRIAL BEHAVIOR AND TRACKMAKING ABILITY WITHIN THE CHIROPTERA ______________________________ Chairperson: Stephen T. Hasiotis ______________________________ Co-chairperson: David A. Burnham Date Approved: April 8, 2016 ii ABSTRACT Among living mammals, bats (Chiroptera) are second only to rodents in total number of species with over 1100 currently known. Extant bat species occupy many trophic niches and feeding habits, including frugivores (fruit eaters), insectivores (insect eaters), nectarivores (nectar and pollen-eaters), carnivores (predators of small terrestrial vertebrates), piscivores (fish eaters), sanguinivores (blood eaters), and omnivores (eat animals and plant material). Modern bats also demonstrate a wide range of terrestrial abilities while feeding, including: (1) those that primarily feed at or near ground level, such as the common vampire bat (Desmodus rotundus) and the New Zealand short-tailed bat (Mystacina tuberculata); (2) those rarely observed to feed from or otherwise spend time on the ground; and (3) many intermediate forms that demonstrate terrestrial competency without an obvious ecological basis. The variation in chiropteran terrestrial ability has been hypothesized to be constrained by the morphology of the pelvis and hindlimbs into what are termed types 1, 2, and 3 bats. -
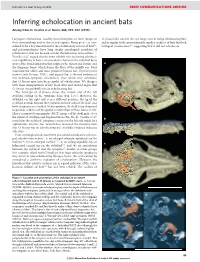
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

BIO 313 ANIMAL ECOLOGY Corrected
NATIONAL OPEN UNIVERSITY OF NIGERIA SCHOOL OF SCIENCE AND TECHNOLOGY COURSE CODE: BIO 314 COURSE TITLE: ANIMAL ECOLOGY 1 BIO 314: ANIMAL ECOLOGY Team Writers: Dr O.A. Olajuyigbe Department of Biology Adeyemi Colledge of Education, P.M.B. 520, Ondo, Ondo State Nigeria. Miss F.C. Olakolu Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. Mrs H.O. Omogoriola Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. EDITOR: Mrs Ajetomobi School of Agricultural Sciences Lagos State Polytechnic Ikorodu, Lagos 2 BIO 313 COURSE GUIDE Introduction Animal Ecology (313) is a first semester course. It is a two credit unit elective course which all students offering Bachelor of Science (BSc) in Biology can take. Animal ecology is an important area of study for scientists. It is the study of animals and how they related to each other as well as their environment. It can also be defined as the scientific study of interactions that determine the distribution and abundance of organisms. Since this is a course in animal ecology, we will focus on animals, which we will define fairly generally as organisms that can move around during some stages of their life and that must feed on other organisms or their products. There are various forms of animal ecology. This includes: • Behavioral ecology, the study of the behavior of the animals with relation to their environment and others • Population ecology, the study of the effects on the population of these animals • Marine ecology is the scientific study of marine-life habitat, populations, and interactions among organisms and the surrounding environment including their abiotic (non-living physical and chemical factors that affect the ability of organisms to survive and reproduce) and biotic factors (living things or the materials that directly or indirectly affect an organism in its environment). -

Origin and Beyond
EVOLUTION ORIGIN ANDBEYOND Gould, who alerted him to the fact the Galapagos finches ORIGIN AND BEYOND were distinct but closely related species. Darwin investigated ALFRED RUSSEL WALLACE (1823–1913) the breeding and artificial selection of domesticated animals, and learned about species, time, and the fossil record from despite the inspiration and wealth of data he had gathered during his years aboard the Alfred Russel Wallace was a school teacher and naturalist who gave up teaching the anatomist Richard Owen, who had worked on many of to earn his living as a professional collector of exotic plants and animals from beagle, darwin took many years to formulate his theory and ready it for publication – Darwin’s vertebrate specimens and, in 1842, had “invented” the tropics. He collected extensively in South America, and from 1854 in the so long, in fact, that he was almost beaten to publication. nevertheless, when it dinosaurs as a separate category of reptiles. islands of the Malay archipelago. From these experiences, Wallace realized By 1842, Darwin’s evolutionary ideas were sufficiently emerged, darwin’s work had a profound effect. that species exist in variant advanced for him to produce a 35-page sketch and, by forms and that changes in 1844, a 250-page synthesis, a copy of which he sent in 1847 the environment could lead During a long life, Charles After his five-year round the world voyage, Darwin arrived Darwin saw himself largely as a geologist, and published to the botanist, Joseph Dalton Hooker. This trusted friend to the loss of any ill-adapted Darwin wrote numerous back at the family home in Shrewsbury on 5 October 1836. -

Proquest Dissertations
Universite de Montreal Chimeres, donnees manquantes et congruence: validation de differentes methodes par simulations et application a la phylogenie des mammiferes par Veronique Campbell Departement de sciences biologiques Faculte des arts et sciences These presentee a la Faculte des etudes superieures en vue de I'obtention du grade de Philosophiae doctor (Ph. D.) en sciences biologiques Aout, 2009 © Veronique Campbell, 200 Library and Archives Bibliotheque et 1*1 Canada Archives Canada Published Heritage Direction du Branch Patrimoine de I'edition 395 Wellington Street 395, rue Wellington Ottawa ON K1A 0N4 OttawaONK1A0N4 Canada Canada Your file Votre reference ISBN: 978-0-494-60679-7 Our file Notre reference ISBN: 978-0-494-60679-7 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library and permettant a la Bibliotheque et Archives Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par I'lnternet, prefer, telecommunication or on the Internet, distribuer et vendre des theses partout dans le loan, distribute and sell theses monde, a des fins commerciales ou autres, sur worldwide, for commercial or non support microforme, papier, electronique et/ou commercial purposes, in microform, autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve la propriete du droit d'auteur ownership and moral rights in this et des droits moraux qui protege cette these. Ni thesis. Neither the thesis nor la these ni des extraits substantiels de celle-ci substantial extracts from it may be ne doivent etre imprimes ou autrement printed or otherwise reproduced reproduits sans son autorisation. -

Anatomical Diversification of a Skeletal Novelty in Bat Feet
ORIGINAL ARTICLE doi:10.1111/evo.13786 Anatomical diversification of a skeletal novelty in bat feet Kathryn E. Stanchak,1,2 Jessica H. Arbour,1 and Sharlene E. Santana1 1Department of Biology and Burke Museum of Natural History and Culture, University of Washington, Seattle, Washington 98195 2E-mail: [email protected] Received December 21, 2018 Accepted May 18, 2019 Neomorphic, membrane-associated skeletal rods are found in disparate vertebrate lineages, but their evolution is poorly under- stood. Here we show that one of these elements—the calcar of bats (Chiroptera)—is a skeletal novelty that has anatomically diversified. Comparisons of evolutionary models of calcar length and corresponding disparity-through-time analyses indicate that the calcar diversified early in the evolutionary history of Chiroptera, as bats phylogenetically diversified after evolving the capac- ity for flight. This interspecific variation in calcar length and its relative proportion to tibia and forearm length is of functional relevance to flight-related behaviors. We also find that the calcar varies in its tissue composition among bats, which might affect its response to mechanical loading. We confirm the presence of a synovial joint at the articulation between the calcar and the cal- caneus in some species, which suggests the calcar has a kinematic functional role. Collectively, this functionally relevant variation suggests that adaptive advantages provided by the calcar led to its anatomical diversification. Our results demonstrate that novel skeletal additions can become integrated into vertebrate body plans and subsequently evolve into a variety of forms, potentially impacting clade diversification by expanding the available morphological space into which organisms can evolve. -

The Evolution of Echolocation in Bats: a Comparative Approach
The evolution of echolocation in bats: a comparative approach Alanna Collen A thesis submitted for the degree of Doctor of Philosophy from the Department of Genetics, Evolution and Environment, University College London. November 2012 Declaration Declaration I, Alanna Collen (née Maltby), confirm that the work presented in this thesis is my own. Where information has been derived from other sources, this is indicated in the thesis, and below: Chapter 1 This chapter is published in the Handbook of Mammalian Vocalisations (Maltby, Jones, & Jones) as a first authored book chapter with Gareth Jones and Kate Jones. Gareth Jones provided the research for the genetics section, and both Kate Jones and Gareth Jones providing comments and edits. Chapter 2 The raw echolocation call recordings in EchoBank were largely made and contributed by members of the ‘Echolocation Call Consortium’ (see full list in Chapter 2). The R code for the diversity maps was provided by Kamran Safi. Custom adjustments were made to the computer program SonoBat by developer Joe Szewczak, Humboldt State University, in order to select echolocation calls for measurement. Chapter 3 The supertree construction process was carried out using Perl scripts developed and provided by Olaf Bininda-Emonds, University of Oldenburg, and the supertree was run and dated by Olaf Bininda-Emonds. The source trees for the Pteropodidae were collected by Imperial College London MSc student Christina Ravinet. Chapter 4 Rob Freckleton, University of Sheffield, and Luke Harmon, University of Idaho, helped with R code implementation. 2 Declaration Chapter 5 Luke Harmon, University of Idaho, helped with R code implementation. Chapter 6 Joseph W. -

Comparative Cognition and Social Learning in Bats
Learn and Let Learn: Comparative Cognition and Social Learning in Bats Dissertation zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften vorgelegt von Theresa Maria Anna Clarin an der Mathematisch-Naturwissenschaftliche Sektion Fachbereich Biologie Tag der mündlichen Prüfung: 16.07.2014 1. Referent: Prof. Dr. Martin Wikelski 2. Referent: Assistant Prof. Dr. John Ratcliffe for PD Dr. Björn M. Siemers Die geistigen Fähigkeiten der Flatterthiere sind keineswegs so gering, als man gern annehmen möchte, und strafen den auf ziemliche Geistesarmuth hindeutenden Gesichtsausdruck Lügen. [...] Alle Flatterthiere zeichnen sich durch einen ziemlich hohen Grad von Gedächtnis und einige sogar durch verständige Ueberlegung aus. (Brehm , A.E. 1864 . Illustrirtes Thierleben: eine allgemeine Kunde des Thierreichs ). The intellectual abilities of the bats are by no means as low as one would think and give the lie to the expression of their faces, which is indicative of a certain poverty of mind. […] All bats stand out by a rather high degree of memory and some even by the presence of reasoning powers. TABLE OF CONTENTS SUMMARY ...................................................................................................................................................... 1 ZUSAMMENFASSUNG (GERMAN SUMMARY) ................................................................................................. 3 GENERAL INTRODUCTION .............................................................................................................................. -

Molecular Evolution and the Sensory Biology of Bats
REVIEW ARTICLE published: 30 May 2013 doi: 10.3389/fphys.2013.00117 From the ultrasonic to the infrared: molecular evolution and the sensory biology of bats Gareth Jones 1*, Emma C. Teeling 2 and Stephen J. Rossiter 3 1 School of Biological Sciences, University of Bristol, Bristol, UK 2 UCD School of Biology and Environmental Science, University College Dublin, Dublin, Ireland 3 School of Biological and Chemical Sciences, Queen Mary, University of London, London, UK Edited by: Great advances have been made recently in understanding the genetic basis of the Cynthia F. Moss, University of sensory biology of bats. Research has focused on the molecular evolution of candidate Maryland, USA sensory genes, genes with known functions [e.g., olfactory receptor (OR) genes] and Reviewed by: genes identified from mutations associated with sensory deficits (e.g., blindness and Brock Fenton, University of Western Ontario, Canada deafness). For example, the FoxP2 gene, underpinning vocal behavior and sensorimotor Gerald S. Wilkinson, University of coordination, has undergone diversification in bats, while several genes associated with Maryland, USA audition show parallel amino acid substitutions in unrelated lineages of echolocating Nancy Simmons, American bats and, in some cases, in echolocating dolphins, representing a classic case of Museum of Natural History, USA convergent molecular evolution. Vision genes encoding the photopigments rhodopsin *Correspondence: Gareth Jones, School of Biological and the long-wave sensitive opsin are functional in bats, while that encoding the Sciences, University of Bristol, short-wave sensitive opsin has lost functionality in rhinolophoid bats using high-duty Woodland Road, Bristol BS8 1UG, cycle laryngeal echolocation, suggesting a sensory trade-off between investment in UK vision and echolocation. -

To Scream Or to Listen? Prey Detection and Discrimination in Animal-Eating Bats
96 P.L. Jones et al. Fig. 4.1 Current phylogeny for bats (Jones and Teeling 2006 ). To the right of each family name, a Substrate Gleaner icon indicates that, in our opinion, this family is characterized by bat species that rely primarily on a gleaning strategy; all or most of which also take some prey by aerial hawk- ing. An Aerial Hawker icon indicates that the family consists of species most of which primarily use a hawking strategy but includes behaviorally fl exible species. Crasoenycteridae comprises a single behaviorally fl exible species. A Frugivore/Nectivore icon indicates a family comprised solely or partially of frugivorous and nectivorous species Each scenario, however, still suggests the same general story (Figure 4.1 ). That is, early laryngeal echolocating bats used powered fl ight, hunted animals, and took them in the air. The latter supposition is supported by the fact that most early fossil bats (~50 million years old) had wing designs suited for aerially hawking and not 4 Prey Detection and Discrimination in Animal-Eating Bats 97 like those of modern gleaners (Simmons and Geisler 1998 ; Safi et al. 2005 ). Therefore, we suppose that while something akin to gleaning may have characterized proto-bats and the very earliest of bats, this trait may have been subsequently lost, at least as a primary means of prey capture, as bats evolved more sophisticated laryngeal echolocation and longer, narrower wings, and then gleaning evolved inde- pendently again multiple times (Simmons and Geisler 1998 ) (Figure 4.1 ). However, while this idea is supported by fossil evidence (Simmons and Geisler 1998 ) and phylogenetic trait reconstructions (Safi et al. -

Integrated Fossil and Molecular Data Reconstruct Bat Echolocation
Integrated fossil and molecular data reconstruct bat echolocation Mark S. Springer†‡, Emma C. Teeling†§, Ole Madsen¶, Michael J. Stanhope§ʈ, and Wilfried W. de Jong¶** †Department of Biology, University of California, Riverside, CA 92521; §Queen’s University of Belfast, Biology and Biochemistry, 97 Lisburn Road, Belfast BT9 7BL, United Kingdom; ¶Department of Biochemistry, University of Nijmegen, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands; ʈBioinformatics, SmithKline Beecham Pharmaceuticals, 1250 South Collegeville Road, UP1345, Collegeville, PA 19426; and **Institute for Biodiversity and Ecosystem Dynamics, 1090 GT Amsterdam, The Netherlands Edited by David Pilbeam, Harvard University, Cambridge, MA, and approved March 26, 2001 (received for review November 21, 2000) Molecular and morphological data have important roles in illumi- tulates a sister-group relationship between flying lemurs and bats nating evolutionary history. DNA data often yield well resolved (6). Although some authors have questioned bat monophyly phylogenies for living taxa, but are generally unattainable for based on evidence from the penis and nervous system (7, 8), the fossils. A distinct advantage of morphology is that some types of bulk of morphological data supports bat monophyly (9). Among morphological data may be collected for extinct and extant taxa. living Chiroptera, morphological data provide strong support for Fossils provide a unique window on evolutionary history and may the reciprocal monophyly of megachiropterans (Old World fruit preserve combinations