Harrison:Layout 1.Qxd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bat Echolocation Research a Handbook for Planning and Conducting Acoustic Studies Second Edition
Bat Echolocation Research A handbook for planning and conducting acoustic studies Second Edition Erin E. Fraser, Alexander Silvis, R. Mark Brigham, and Zenon J. Czenze EDITORS Bat Echolocation Research A handbook for planning and conducting acoustic studies Second Edition Editors Erin E. Fraser, Alexander Silvis, R. Mark Brigham, and Zenon J. Czenze Citation Fraser et al., eds. 2020. Bat Echolocation Research: A handbook for planning and conducting acoustic studies. Second Edition. Bat Conservation International. Austin, Texas, USA. Tucson, Arizona 2020 This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License ii Table of Contents Table of Figures ....................................................................................................................................................................... vi Table of Tables ........................................................................................................................................................................ vii Contributing Authors .......................................................................................................................................................... viii Dedication…… .......................................................................................................................................................................... xi Foreword…….. .......................................................................................................................................................................... -

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -
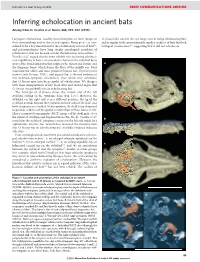
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

BIO 313 ANIMAL ECOLOGY Corrected
NATIONAL OPEN UNIVERSITY OF NIGERIA SCHOOL OF SCIENCE AND TECHNOLOGY COURSE CODE: BIO 314 COURSE TITLE: ANIMAL ECOLOGY 1 BIO 314: ANIMAL ECOLOGY Team Writers: Dr O.A. Olajuyigbe Department of Biology Adeyemi Colledge of Education, P.M.B. 520, Ondo, Ondo State Nigeria. Miss F.C. Olakolu Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. Mrs H.O. Omogoriola Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. EDITOR: Mrs Ajetomobi School of Agricultural Sciences Lagos State Polytechnic Ikorodu, Lagos 2 BIO 313 COURSE GUIDE Introduction Animal Ecology (313) is a first semester course. It is a two credit unit elective course which all students offering Bachelor of Science (BSc) in Biology can take. Animal ecology is an important area of study for scientists. It is the study of animals and how they related to each other as well as their environment. It can also be defined as the scientific study of interactions that determine the distribution and abundance of organisms. Since this is a course in animal ecology, we will focus on animals, which we will define fairly generally as organisms that can move around during some stages of their life and that must feed on other organisms or their products. There are various forms of animal ecology. This includes: • Behavioral ecology, the study of the behavior of the animals with relation to their environment and others • Population ecology, the study of the effects on the population of these animals • Marine ecology is the scientific study of marine-life habitat, populations, and interactions among organisms and the surrounding environment including their abiotic (non-living physical and chemical factors that affect the ability of organisms to survive and reproduce) and biotic factors (living things or the materials that directly or indirectly affect an organism in its environment). -

Origin and Beyond
EVOLUTION ORIGIN ANDBEYOND Gould, who alerted him to the fact the Galapagos finches ORIGIN AND BEYOND were distinct but closely related species. Darwin investigated ALFRED RUSSEL WALLACE (1823–1913) the breeding and artificial selection of domesticated animals, and learned about species, time, and the fossil record from despite the inspiration and wealth of data he had gathered during his years aboard the Alfred Russel Wallace was a school teacher and naturalist who gave up teaching the anatomist Richard Owen, who had worked on many of to earn his living as a professional collector of exotic plants and animals from beagle, darwin took many years to formulate his theory and ready it for publication – Darwin’s vertebrate specimens and, in 1842, had “invented” the tropics. He collected extensively in South America, and from 1854 in the so long, in fact, that he was almost beaten to publication. nevertheless, when it dinosaurs as a separate category of reptiles. islands of the Malay archipelago. From these experiences, Wallace realized By 1842, Darwin’s evolutionary ideas were sufficiently emerged, darwin’s work had a profound effect. that species exist in variant advanced for him to produce a 35-page sketch and, by forms and that changes in 1844, a 250-page synthesis, a copy of which he sent in 1847 the environment could lead During a long life, Charles After his five-year round the world voyage, Darwin arrived Darwin saw himself largely as a geologist, and published to the botanist, Joseph Dalton Hooker. This trusted friend to the loss of any ill-adapted Darwin wrote numerous back at the family home in Shrewsbury on 5 October 1836. -

Summer Indiana Bat Ecology in the Southern Appalachians
SUMMER INDIANA BAT ECOLOGY IN THE SOUTHERN APPALACHIANS: AN INVESTIGATION OF THERMOREGULATION STRATEGIES AND LANDSCAPE SCALE ROOST SELECTION _______________________ A thesis Presented to The College of Graduate and Professional Studies Department of Biology Indiana State University Terre Haute, Indiana ______________________ In Partial Fulfillment of the Requirements for the Degree Master’s in Biology _______________________ by Kristina Hammond August 2013 Kristina Hammond 2013 Keywords: Myotis sodalis, Indiana bat, thermoregulation, MaxENT, landscape, roosting ecology ii COMMITTEE MEMBERS Committee Chair: Joy O’Keefe, PhD Assistant Professor of Biology Indiana State University Committee Member: George Bakken, PhD Professor of Biology Indiana State University Committee Member: Stephen Aldrich, PhD Assistant Professor of Geography Indiana State University Committee Member: Susan Loeb, PhD Research Ecologist USDA Forest Service, Southern Research Station & Clemson University iii ABSTRACT In the southern Appalachians there are few data on the roost ecology of the federally endangered Indiana bat (Myotis sodalis). During 2008-2012, we investigated roosting ecology of the Indiana bat in ~280,000 ha in the Great Smoky Mountains National Park, Cherokee National Forest, and Nantahala National Forest in the southern Appalachians Mountains of Tennessee and North Carolina. We investigated 2 aspects of the Indiana bat’s roosting ecology: thermoregulation and the extrinsic factors that influence body temperature, and landscape-scale roost selection. To investigate thermoregulation of bats at roost, we used data gathered in 2012 from 6 female Indiana bats (5 adults and 1 juvenile) to examine how reproductive condition, group size, roost characteristics, air temperature, and barometric pressure related to body temperature of roosting bats. We found that air temperature was the primary factor correlated with bats’ body temperatures while at roost (P < 0.01), with few differences detected among reproductive classes in terms of thermoregulatory strategies. -

České Vernakulární Jmenosloví Netopýrů. I. Návrh Úplného Jmenosloví
Vespertilio 13–14: 263–308, 2010 ISSN 1213-6123 České vernakulární jmenosloví netopýrů. I. Návrh úplného jmenosloví Petr Benda zoologické oddělení PM, Národní museum, Václavské nám. 68, CZ–115 79 Praha 1, Česko; katedra zoologie, PřF University Karlovy, Viničná 7, CZ–128 44 Praha 2, Česko; [email protected] Czech vernacular nomenclature of bats. I. Proposal of complete nomenclature. The first and also the last complete Czech vernacular nomenclature of bats was proposed by Presl (1834), who created names for three suborders (families), 31 genera and 110 species of bats (along with names for all other then known mammals). However, his nomenclature is almost forgotten and is not in common use any more. Although more or less representative Czech nomenclatures of bats were later proposed several times, they were never complete. The most comprehensive nomenclature was proposed by Anděra (1999), who gave names for all supra-generic taxa (mostly homonymial) and for 284 species within the order Chiroptera (ca. 31% of species names compiled by Koopman 1993). A new proposal of a complete Czech nomenclature of bats is given in the Appendix. The review of bat taxonomy by Simmons (2005) was adopted and complemented by several new taxa proposed in the last years (altogether ca. 1200 names). For all taxa, a Czech name (in binomial structure for species following the scientific zoological nomenclature) was adopted from previous vernacular nomenclatures or created as a new name, with an idea to give distinct original Czech generic names to representatives of all families, in cases of species- -rich families also of subfamilies or tribes. -

The Evolution of Echolocation in Bats: a Comparative Approach
The evolution of echolocation in bats: a comparative approach Alanna Collen A thesis submitted for the degree of Doctor of Philosophy from the Department of Genetics, Evolution and Environment, University College London. November 2012 Declaration Declaration I, Alanna Collen (née Maltby), confirm that the work presented in this thesis is my own. Where information has been derived from other sources, this is indicated in the thesis, and below: Chapter 1 This chapter is published in the Handbook of Mammalian Vocalisations (Maltby, Jones, & Jones) as a first authored book chapter with Gareth Jones and Kate Jones. Gareth Jones provided the research for the genetics section, and both Kate Jones and Gareth Jones providing comments and edits. Chapter 2 The raw echolocation call recordings in EchoBank were largely made and contributed by members of the ‘Echolocation Call Consortium’ (see full list in Chapter 2). The R code for the diversity maps was provided by Kamran Safi. Custom adjustments were made to the computer program SonoBat by developer Joe Szewczak, Humboldt State University, in order to select echolocation calls for measurement. Chapter 3 The supertree construction process was carried out using Perl scripts developed and provided by Olaf Bininda-Emonds, University of Oldenburg, and the supertree was run and dated by Olaf Bininda-Emonds. The source trees for the Pteropodidae were collected by Imperial College London MSc student Christina Ravinet. Chapter 4 Rob Freckleton, University of Sheffield, and Luke Harmon, University of Idaho, helped with R code implementation. 2 Declaration Chapter 5 Luke Harmon, University of Idaho, helped with R code implementation. Chapter 6 Joseph W. -

To Scream Or to Listen? Prey Detection and Discrimination in Animal-Eating Bats
96 P.L. Jones et al. Fig. 4.1 Current phylogeny for bats (Jones and Teeling 2006 ). To the right of each family name, a Substrate Gleaner icon indicates that, in our opinion, this family is characterized by bat species that rely primarily on a gleaning strategy; all or most of which also take some prey by aerial hawk- ing. An Aerial Hawker icon indicates that the family consists of species most of which primarily use a hawking strategy but includes behaviorally fl exible species. Crasoenycteridae comprises a single behaviorally fl exible species. A Frugivore/Nectivore icon indicates a family comprised solely or partially of frugivorous and nectivorous species Each scenario, however, still suggests the same general story (Figure 4.1 ). That is, early laryngeal echolocating bats used powered fl ight, hunted animals, and took them in the air. The latter supposition is supported by the fact that most early fossil bats (~50 million years old) had wing designs suited for aerially hawking and not 4 Prey Detection and Discrimination in Animal-Eating Bats 97 like those of modern gleaners (Simmons and Geisler 1998 ; Safi et al. 2005 ). Therefore, we suppose that while something akin to gleaning may have characterized proto-bats and the very earliest of bats, this trait may have been subsequently lost, at least as a primary means of prey capture, as bats evolved more sophisticated laryngeal echolocation and longer, narrower wings, and then gleaning evolved inde- pendently again multiple times (Simmons and Geisler 1998 ) (Figure 4.1 ). However, while this idea is supported by fossil evidence (Simmons and Geisler 1998 ) and phylogenetic trait reconstructions (Safi et al. -

Integrated Fossil and Molecular Data Reconstruct Bat Echolocation
Integrated fossil and molecular data reconstruct bat echolocation Mark S. Springer†‡, Emma C. Teeling†§, Ole Madsen¶, Michael J. Stanhope§ʈ, and Wilfried W. de Jong¶** †Department of Biology, University of California, Riverside, CA 92521; §Queen’s University of Belfast, Biology and Biochemistry, 97 Lisburn Road, Belfast BT9 7BL, United Kingdom; ¶Department of Biochemistry, University of Nijmegen, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands; ʈBioinformatics, SmithKline Beecham Pharmaceuticals, 1250 South Collegeville Road, UP1345, Collegeville, PA 19426; and **Institute for Biodiversity and Ecosystem Dynamics, 1090 GT Amsterdam, The Netherlands Edited by David Pilbeam, Harvard University, Cambridge, MA, and approved March 26, 2001 (received for review November 21, 2000) Molecular and morphological data have important roles in illumi- tulates a sister-group relationship between flying lemurs and bats nating evolutionary history. DNA data often yield well resolved (6). Although some authors have questioned bat monophyly phylogenies for living taxa, but are generally unattainable for based on evidence from the penis and nervous system (7, 8), the fossils. A distinct advantage of morphology is that some types of bulk of morphological data supports bat monophyly (9). Among morphological data may be collected for extinct and extant taxa. living Chiroptera, morphological data provide strong support for Fossils provide a unique window on evolutionary history and may the reciprocal monophyly of megachiropterans (Old World fruit preserve combinations -

Bats of the SA Murray Region
Community Bat Monitoring Program with the Mid Murray LAP The Mid Murray Local Action Planning Committee (Mid Murray LAP) BATS has been coordinating a Community Bat Monitoring Program since 2003. This program has been part of the 'Bats for Biodiversity' project initiated by the Mt Pleasant Natural Resource Centre and the Upper of South Australia's Murray Region Torrens Landcare Group. Over the years, many landholders have borrowed an Anabat detector to record the species they have on Bats are one of the largest groups of mammals: with approximately 1000 their properties. species, they make up nearly a quarter of the world's mammal species. There are around 90 species in Australia. Bats are unlike other small mammals in that they can live for a long time, at least 10 years, and are the only mammal that have the ability of sustainable flight. W S N Bats belong to the Order Chiroptera, which means Morgan hand-wing. Microchiroptera (microbats) and Harp nets Megachiroptera (megabats) are the two main groups (suborders). There are 16 species of microbats found Renmark in South Australia's Murray Region. Waikerie An Anabat detector is electronic equipment that Berri The South Australia's Murray Region is defined as the allows the passive monitoring of bats by detecting Blanchetown Catchment area of the Murray River from the South and recording their ultrasonic echolocation calls. Australian border to the Murray Mouth. The echolocation calls of bats are unique to each Loxton species and can be used to identify the bats. All of the bat species in South Australia's Murray Valley are insectivorous, with the exception of the The Mid Murray LAP's Community Bat Monitoring endangered Myotis macropus that also catches fish program includes the Anabat detectors being used with its large hind feet. -

A LIST of the VERTEBRATES of SOUTH AUSTRALIA
A LIST of the VERTEBRATES of SOUTH AUSTRALIA updates. for Edition 4th Editors See A.C. Robinson K.D. Casperson Biological Survey and Research Heritage and Biodiversity Division Department for Environment and Heritage, South Australia M.N. Hutchinson South Australian Museum Department of Transport, Urban Planning and the Arts, South Australia 2000 i EDITORS A.C. Robinson & K.D. Casperson, Biological Survey and Research, Biological Survey and Research, Heritage and Biodiversity Division, Department for Environment and Heritage. G.P.O. Box 1047, Adelaide, SA, 5001 M.N. Hutchinson, Curator of Reptiles and Amphibians South Australian Museum, Department of Transport, Urban Planning and the Arts. GPO Box 234, Adelaide, SA 5001updates. for CARTOGRAPHY AND DESIGN Biological Survey & Research, Heritage and Biodiversity Division, Department for Environment and Heritage Edition Department for Environment and Heritage 2000 4thISBN 0 7308 5890 1 First Edition (edited by H.J. Aslin) published 1985 Second Edition (edited by C.H.S. Watts) published 1990 Third Edition (edited bySee A.C. Robinson, M.N. Hutchinson, and K.D. Casperson) published 2000 Cover Photograph: Clockwise:- Western Pygmy Possum, Cercartetus concinnus (Photo A. Robinson), Smooth Knob-tailed Gecko, Nephrurus levis (Photo A. Robinson), Painted Frog, Neobatrachus pictus (Photo A. Robinson), Desert Goby, Chlamydogobius eremius (Photo N. Armstrong),Osprey, Pandion haliaetus (Photo A. Robinson) ii _______________________________________________________________________________________ CONTENTS