Wrap.Warwick.Ac.Uk/88401
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Neoichnology of Bats: Morphological, Ecological, and Phylogenetic Influences on Terrestrial Behavior and Trackmaking Ability Within the Chiroptera
NEOICHNOLOGY OF BATS: MORPHOLOGICAL, ECOLOGICAL, AND PHYLOGENETIC INFLUENCES ON TERRESTRIAL BEHAVIOR AND TRACKMAKING ABILITY WITHIN THE CHIROPTERA BY MATTHEW FRAZER JONES Submitted to the graduate degree program in Geology and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Master of Science. Advisory Committee: ______________________________ Chairperson Stephen T. Hasiotis ______________________________ Co-chair David A. Burnham ______________________________ Robert M. Timm Date Defended: April 8, 2016 The Thesis Committee for MATTHEW FRAZER JONES certifies that this is the approved version of the following thesis: NEOICHNOLOGY OF BATS: MORPHOLOGICAL, ECOLOGICAL, AND PHYLOGENETIC INFLUENCES ON TERRESTRIAL BEHAVIOR AND TRACKMAKING ABILITY WITHIN THE CHIROPTERA ______________________________ Chairperson: Stephen T. Hasiotis ______________________________ Co-chairperson: David A. Burnham Date Approved: April 8, 2016 ii ABSTRACT Among living mammals, bats (Chiroptera) are second only to rodents in total number of species with over 1100 currently known. Extant bat species occupy many trophic niches and feeding habits, including frugivores (fruit eaters), insectivores (insect eaters), nectarivores (nectar and pollen-eaters), carnivores (predators of small terrestrial vertebrates), piscivores (fish eaters), sanguinivores (blood eaters), and omnivores (eat animals and plant material). Modern bats also demonstrate a wide range of terrestrial abilities while feeding, including: (1) those that primarily feed at or near ground level, such as the common vampire bat (Desmodus rotundus) and the New Zealand short-tailed bat (Mystacina tuberculata); (2) those rarely observed to feed from or otherwise spend time on the ground; and (3) many intermediate forms that demonstrate terrestrial competency without an obvious ecological basis. The variation in chiropteran terrestrial ability has been hypothesized to be constrained by the morphology of the pelvis and hindlimbs into what are termed types 1, 2, and 3 bats. -

Correspondence Between Ecomorphotype and Use of Arthropod Resources by Bats of the Genus Myotis
Journal of Mammalogy, 99(3):659–667, 2018 DOI:10.1093/jmammal/gyy049 Published online May 14, 2018 Correspondence between ecomorphotype and use of arthropod resources by bats of the genus Myotis Downloaded from https://academic.oup.com/jmammal/article-abstract/99/3/659/4996004 by University of Connecticut user on 17 September 2018 CINTYA A. SEGURA-TRUJILLO, MICHAEL R. WILLIG, AND SERGIO TICUL ÁLVAREZ-CASTAÑEDA* Centro de Investigaciones Biológicas del Noroeste, S.C. Av. Instituto Politécnico Nacional 195, Playa Palo de Santa Rita Sur, La Paz 23096, Baja California Sur, México (CAS-T, STA-C) Center for Environmental Sciences & Engineering and Department of Ecology & Evolutionary Biology, University of Connecticut, 3107 Horsebarn Hill Road, Storrs, CT 06269-4210, USA (MRW) * Correspondent: [email protected] Arthropods are the primary dietary constituents of species of the genus Myotis. The genus comprises 3 ecomorphotypes of polyphyletic origin, each associated with a different foraging strategy: aerial, trawling, and gleaning, related to the subgenera Selysius, Leuconoe, and Myotis, respectively. We explored the extent to which differences in diet characterized these ecomorphotypes. Based on a broad review of the literature, we classified the diet of species of Myotis based on hardness and vagility of consumed prey. A significant negative relationship was found between the percent volume consumption of hard and soft arthropods and between the aerodynamic characteristics of fast- and slow-flying prey. A cluster analysis yielded 3 groups of Myotis species based on their diet: 1) those for which hard prey represent more than 80% of the volume of excreta; 2) those for which hard prey represent 45% to 80% of the volume of excreta; and 3) those for which hard prey represent less than 45% of the volume of excreta. -

Four Species in One: Multigene Analyses Reveal Phylogenetic
Published by Associazione Teriologica Italiana Volume 29 (1): 111–121, 2018 Hystrix, the Italian Journal of Mammalogy Available online at: http://www.italian-journal-of-mammalogy.it doi:10.4404/hystrix–00017-2017 Research Article Four species in one: multigene analyses reveal phylogenetic patterns within Hardwicke’s woolly bat, Kerivoula hardwickii-complex (Chiroptera, Vespertilionidae) in Asia Vuong Tan Tu1,2,3,4,∗, Alexandre Hassanin1,2,∗, Neil M. Furey5, Nguyen Truong Son3,4, Gábor Csorba6 1Institut de Systématique, Evolution, Biodiversité (ISYEB), UMR 7205 MNHN CNRS UPMC, Muséum national d’Histoire naturelle, Case postale N°51–55, rue Buffon, 75005 Paris, France 2Service de Systématique Moléculaire, UMS 2700, Muséum national d’Histoire naturelle, Case postale N°26–43, rue Cuvier, 75005 Paris, France 3Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet road, Cau Giay district, Hanoi, Vietnam 4Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet road, Cau Giay district, Hanoi, Vietnam 5Fauna & Flora International, Cambodia Programme, 19 Street 360, Boeng Keng Kang 1, Chamkarmorn, Phnom Penh, Cambodia 6Department of Zoology, Hungarian Natural History Museum, Baross u. 13., H-1088, Budapest, Hungary Keywords: Abstract Kerivoulinae Asia We undertook a comparative phylogeographic study using molecular, morphological and morpho- phylogeography metric approaches to address systematic issues in bats of the Kerivoula hardwickii complex in Asia. taxonomy Our phylogenetic reconstructions using DNA sequences of two mitochondrial and seven nuclear cryptic species genes reveal a distinct clade containing four small-sized species (K. hardwickii sensu stricto, K. depressa, K. furva and Kerivoula sp. -
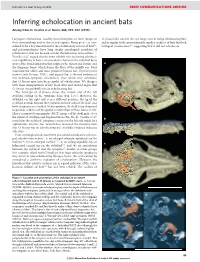
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

BIO 313 ANIMAL ECOLOGY Corrected
NATIONAL OPEN UNIVERSITY OF NIGERIA SCHOOL OF SCIENCE AND TECHNOLOGY COURSE CODE: BIO 314 COURSE TITLE: ANIMAL ECOLOGY 1 BIO 314: ANIMAL ECOLOGY Team Writers: Dr O.A. Olajuyigbe Department of Biology Adeyemi Colledge of Education, P.M.B. 520, Ondo, Ondo State Nigeria. Miss F.C. Olakolu Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. Mrs H.O. Omogoriola Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. EDITOR: Mrs Ajetomobi School of Agricultural Sciences Lagos State Polytechnic Ikorodu, Lagos 2 BIO 313 COURSE GUIDE Introduction Animal Ecology (313) is a first semester course. It is a two credit unit elective course which all students offering Bachelor of Science (BSc) in Biology can take. Animal ecology is an important area of study for scientists. It is the study of animals and how they related to each other as well as their environment. It can also be defined as the scientific study of interactions that determine the distribution and abundance of organisms. Since this is a course in animal ecology, we will focus on animals, which we will define fairly generally as organisms that can move around during some stages of their life and that must feed on other organisms or their products. There are various forms of animal ecology. This includes: • Behavioral ecology, the study of the behavior of the animals with relation to their environment and others • Population ecology, the study of the effects on the population of these animals • Marine ecology is the scientific study of marine-life habitat, populations, and interactions among organisms and the surrounding environment including their abiotic (non-living physical and chemical factors that affect the ability of organisms to survive and reproduce) and biotic factors (living things or the materials that directly or indirectly affect an organism in its environment). -

Origin and Beyond
EVOLUTION ORIGIN ANDBEYOND Gould, who alerted him to the fact the Galapagos finches ORIGIN AND BEYOND were distinct but closely related species. Darwin investigated ALFRED RUSSEL WALLACE (1823–1913) the breeding and artificial selection of domesticated animals, and learned about species, time, and the fossil record from despite the inspiration and wealth of data he had gathered during his years aboard the Alfred Russel Wallace was a school teacher and naturalist who gave up teaching the anatomist Richard Owen, who had worked on many of to earn his living as a professional collector of exotic plants and animals from beagle, darwin took many years to formulate his theory and ready it for publication – Darwin’s vertebrate specimens and, in 1842, had “invented” the tropics. He collected extensively in South America, and from 1854 in the so long, in fact, that he was almost beaten to publication. nevertheless, when it dinosaurs as a separate category of reptiles. islands of the Malay archipelago. From these experiences, Wallace realized By 1842, Darwin’s evolutionary ideas were sufficiently emerged, darwin’s work had a profound effect. that species exist in variant advanced for him to produce a 35-page sketch and, by forms and that changes in 1844, a 250-page synthesis, a copy of which he sent in 1847 the environment could lead During a long life, Charles After his five-year round the world voyage, Darwin arrived Darwin saw himself largely as a geologist, and published to the botanist, Joseph Dalton Hooker. This trusted friend to the loss of any ill-adapted Darwin wrote numerous back at the family home in Shrewsbury on 5 October 1836. -

Molecular Phylogeny of Mobatviruses (Hantaviridae) in Myanmar and Vietnam
viruses Article Molecular Phylogeny of Mobatviruses (Hantaviridae) in Myanmar and Vietnam Satoru Arai 1, Fuka Kikuchi 1,2, Saw Bawm 3 , Nguyễn Trường Sơn 4,5, Kyaw San Lin 6, Vương Tân Tú 4,5, Keita Aoki 1,7, Kimiyuki Tsuchiya 8, Keiko Tanaka-Taya 1, Shigeru Morikawa 9, Kazunori Oishi 1 and Richard Yanagihara 10,* 1 Infectious Disease Surveillance Center, National Institute of Infectious Diseases, Tokyo 162-8640, Japan; [email protected] (S.A.); [email protected] (F.K.); [email protected] (K.A.); [email protected] (K.T.-T.); [email protected] (K.O.) 2 Department of Chemistry, Faculty of Science, Tokyo University of Science, Tokyo 162-8601, Japan 3 Department of Pharmacology and Parasitology, University of Veterinary Science, Yezin, Nay Pyi Taw 15013, Myanmar; [email protected] 4 Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, Hanoi, Vietnam; [email protected] (N.T.S.); [email protected] (V.T.T.) 5 Graduate University of Science and Technology, Vietnam Academy of Science and Technology, Hanoi, Vietnam 6 Department of Aquaculture and Aquatic Disease, University of Veterinary Science, Yezin, Nay Pyi Taw 15013, Myanmar; [email protected] 7 Department of Liberal Arts, Faculty of Science, Tokyo University of Science, Tokyo 162-8601, Japan 8 Laboratory of Bioresources, Applied Biology Co., Ltd., Tokyo 107-0062, Japan; [email protected] 9 Department of Veterinary Science, National Institute of Infectious Diseases, Tokyo 162-8640, Japan; [email protected] 10 Pacific Center for Emerging Infectious Diseases Research, John A. -

Inferring the Ecological Niche of Bat Viruses Closely Related to SARS-Cov-2 Using Phylogeographic Analyses of Rhinolophus Specie
www.nature.com/scientificreports OPEN Inferring the ecological niche of bat viruses closely related to SARS‑CoV‑2 using phylogeographic analyses of Rhinolophus species Alexandre Hassanin1,4*, Vuong Tan Tu2,4, Manon Curaudeau1 & Gabor Csorba3 The Severe Acute Respiratory Syndrome coronavirus 2 (SARS‑CoV‑2) is the causal agent of the coronavirus disease 2019 (COVID‑19) pandemic. To date, viruses closely related to SARS‑CoV‑2 have been reported in four bat species: Rhinolophus acuminatus, Rhinolophus afnis, Rhinolophus malayanus, and Rhinolophus shameli. Here, we analysed 343 sequences of the mitochondrial cytochrome c oxidase subunit 1 gene (CO1) from georeferenced bats of the four Rhinolophus species identifed as reservoirs of viruses closely related to SARS‑CoV‑2. Haplotype networks were constructed in order to investigate patterns of genetic diversity among bat populations of Southeast Asia and China. No strong geographic structure was found for the four Rhinolophus species, suggesting high dispersal capacity. The ecological niche of bat viruses closely related to SARS‑CoV‑2 was predicted using the four localities in which bat viruses were recently discovered and the localities where bats showed the same CO1 haplotypes than virus‑positive bats. The ecological niche of bat viruses related to SARS‑CoV was deduced from the localities where bat viruses were previously detected. The results show that the ecological niche of bat viruses related to SARS‑CoV2 includes several regions of mainland Southeast Asia whereas the ecological niche of bat viruses related to SARS‑CoV is mainly restricted to China. In agreement with these results, human populations in Laos, Vietnam, Cambodia, and Thailand appear to be much less afected by the COVID‑19 pandemic than other countries of Southeast Asia. -

Proquest Dissertations
Universite de Montreal Chimeres, donnees manquantes et congruence: validation de differentes methodes par simulations et application a la phylogenie des mammiferes par Veronique Campbell Departement de sciences biologiques Faculte des arts et sciences These presentee a la Faculte des etudes superieures en vue de I'obtention du grade de Philosophiae doctor (Ph. D.) en sciences biologiques Aout, 2009 © Veronique Campbell, 200 Library and Archives Bibliotheque et 1*1 Canada Archives Canada Published Heritage Direction du Branch Patrimoine de I'edition 395 Wellington Street 395, rue Wellington Ottawa ON K1A 0N4 OttawaONK1A0N4 Canada Canada Your file Votre reference ISBN: 978-0-494-60679-7 Our file Notre reference ISBN: 978-0-494-60679-7 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library and permettant a la Bibliotheque et Archives Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par I'lnternet, prefer, telecommunication or on the Internet, distribuer et vendre des theses partout dans le loan, distribute and sell theses monde, a des fins commerciales ou autres, sur worldwide, for commercial or non support microforme, papier, electronique et/ou commercial purposes, in microform, autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve la propriete du droit d'auteur ownership and moral rights in this et des droits moraux qui protege cette these. Ni thesis. Neither the thesis nor la these ni des extraits substantiels de celle-ci substantial extracts from it may be ne doivent etre imprimes ou autrement printed or otherwise reproduced reproduits sans son autorisation. -

Lotus Wind Power Project
Initial Environmental Examination – Appendix H Project Number: 54211-001 March 2021 Document Stage: Draft Viet Nam: Lotus Wind Power Project Prepared by ERM Vietnam for Lien Lap Wind Power Joint Stock Company, Phong Huy Wind Power Joint Stock Company, and Phong Nguyen Wind Power Joint Stock Company as a requirement of the Asian Development Bank. The initial environmental examination is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. Your attention is directed to the “Terms of Use” section of this website. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. Biodiversity survey Wet season report Phong Huy Wind Power Project, Huong Hoa, Quang Tri, Viet Nam 7 July 2020 Prepared by ERM’s Subcontractor for ERM Vietnam Document details Document title Biodiversity survey Wet season report Document subtitle Phong Huy Wind Power Project, Huong Hoa, Quang Tri, Viet Nam Date 7 July 2020 Version 1.0 Author ERM’s Subcontractor Client Name ERM Vietnam Document history Version Revision Author Reviewed by ERM approval to issue Comments Name Date Draft 1.0 Name Name Name 00.00.0000 Text Version: 1.0 Client: ERM Vietnam 7 July 2020 BIODIVERSITY SURVEY WET SEASON REPORT CONTENTS Phong Huy Wind Power Project, Huong Hoa, Quang Tri, Viet Nam CONTENTS 1. -

Index of Handbook of the Mammals of the World. Vol. 9. Bats
Index of Handbook of the Mammals of the World. Vol. 9. Bats A agnella, Kerivoula 901 Anchieta’s Bat 814 aquilus, Glischropus 763 Aba Leaf-nosed Bat 247 aladdin, Pipistrellus pipistrellus 771 Anchieta’s Broad-faced Fruit Bat 94 aquilus, Platyrrhinus 567 Aba Roundleaf Bat 247 alascensis, Myotis lucifugus 927 Anchieta’s Pipistrelle 814 Arabian Barbastelle 861 abae, Hipposideros 247 alaschanicus, Hypsugo 810 anchietae, Plerotes 94 Arabian Horseshoe Bat 296 abae, Rhinolophus fumigatus 290 Alashanian Pipistrelle 810 ancricola, Myotis 957 Arabian Mouse-tailed Bat 164, 170, 176 abbotti, Myotis hasseltii 970 alba, Ectophylla 466, 480, 569 Andaman Horseshoe Bat 314 Arabian Pipistrelle 810 abditum, Megaderma spasma 191 albatus, Myopterus daubentonii 663 Andaman Intermediate Horseshoe Arabian Trident Bat 229 Abo Bat 725, 832 Alberico’s Broad-nosed Bat 565 Bat 321 Arabian Trident Leaf-nosed Bat 229 Abo Butterfly Bat 725, 832 albericoi, Platyrrhinus 565 andamanensis, Rhinolophus 321 arabica, Asellia 229 abramus, Pipistrellus 777 albescens, Myotis 940 Andean Fruit Bat 547 arabicus, Hypsugo 810 abrasus, Cynomops 604, 640 albicollis, Megaerops 64 Andersen’s Bare-backed Fruit Bat 109 arabicus, Rousettus aegyptiacus 87 Abruzzi’s Wrinkle-lipped Bat 645 albipinnis, Taphozous longimanus 353 Andersen’s Flying Fox 158 arabium, Rhinopoma cystops 176 Abyssinian Horseshoe Bat 290 albiventer, Nyctimene 36, 118 Andersen’s Fruit-eating Bat 578 Arafura Large-footed Bat 969 Acerodon albiventris, Noctilio 405, 411 Andersen’s Leaf-nosed Bat 254 Arata Yellow-shouldered Bat 543 Sulawesi 134 albofuscus, Scotoecus 762 Andersen’s Little Fruit-eating Bat 578 Arata-Thomas Yellow-shouldered Talaud 134 alboguttata, Glauconycteris 833 Andersen’s Naked-backed Fruit Bat 109 Bat 543 Acerodon 134 albus, Diclidurus 339, 367 Andersen’s Roundleaf Bat 254 aratathomasi, Sturnira 543 Acerodon mackloti (see A.