Gastro-Intestinal Helminths of Goliath Frogs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
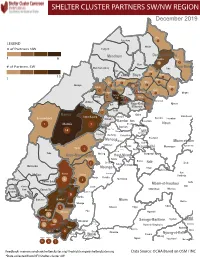
NW SW Presence Map Complete Copy
SHELTER CLUSTER PARTNERS SW/NWMap creation da tREGIONe: 06/12/2018 December 2019 Ako Furu-Awa 1 LEGEND Misaje # of Partners NW Fungom Menchum Donga-Mantung 1 6 Nkambe Nwa 3 1 Bum # of Partners SW Menchum-Valley Ndu Mayo-Banyo Wum Noni 1 Fundong Nkum 15 Boyo 1 1 Njinikom Kumbo Oku 1 Bafut 1 Belo Akwaya 1 3 1 Njikwa Bui Mbven 1 2 Mezam 2 Jakiri Mbengwi Babessi 1 Magba Bamenda Tubah 2 2 Bamenda Ndop Momo 6b 3 4 2 3 Bangourain Widikum Ngie Bamenda Bali 1 Ngo-Ketunjia Njimom Balikumbat Batibo Santa 2 Manyu Galim Upper Bayang Babadjou Malentouen Eyumodjock Wabane Koutaba Foumban Bambo7 tos Kouoptamo 1 Mamfe 7 Lebialem M ouda Noun Batcham Bafoussam Alou Fongo-Tongo 2e 14 Nkong-Ni BafouMssamif 1eir Fontem Dschang Penka-Michel Bamendjou Poumougne Foumbot MenouaFokoué Mbam-et-Kim Baham Djebem Santchou Bandja Batié Massangam Ngambé-Tikar Nguti Koung-Khi 1 Banka Bangou Kekem Toko Kupe-Manenguba Melong Haut-Nkam Bangangté Bafang Bana Bangem Banwa Bazou Baré-Bakem Ndé 1 Bakou Deuk Mundemba Nord-Makombé Moungo Tonga Makénéné Konye Nkongsamba 1er Kon Ndian Tombel Yambetta Manjo Nlonako Isangele 5 1 Nkondjock Dikome Balue Bafia Kumba Mbam-et-Inoubou Kombo Loum Kiiki Kombo Itindi Ekondo Titi Ndikiniméki Nitoukou Abedimo Meme Njombé-Penja 9 Mombo Idabato Bamusso Kumba 1 Nkam Bokito Kumba Mbanga 1 Yabassi Yingui Ndom Mbonge Muyuka Fiko Ngambé 6 Nyanon Lekié West-Coast Sanaga-Maritime Monatélé 5 Fako Dibombari Douala 55 Buea 5e Massock-Songloulou Evodoula Tiko Nguibassal Limbe1 Douala 4e Edéa 2e Okola Limbe 2 6 Douala Dibamba Limbe 3 Douala 6e Wou3rei Pouma Nyong-et-Kellé Douala 6e Dibang Limbe 1 Limbe 2 Limbe 3 Dizangué Ngwei Ngog-Mapubi Matomb Lobo 13 54 1 Feedback: [email protected]/ [email protected] Data Source: OCHA Based on OSM / INC *Data collected from NFI/Shelter cluster 4W. -

Forecasts and Dekadal Climate Alerts for the Period 1St to 10Th June 2021
REPUBLIQUE DU CAMEROUN REPUBLIC OF CAMEROON Paix-Travail-Patrie Peace-Work-Fatherland ----------- ----------- OBSERVATOIRE NATIONAL SUR NATIONAL OBSERVATORY LES CHANGEMENTS CLIMATIQUES ON CLIMATE CHANGE ----------------- ----------------- DIRECTION GENERALE DIRECTORATE GENERAL ----------------- ----------------- ONACC www.onacc.cm; [email protected]; Tel : (+237) 693 370 504 / 654 392 529 BULLETIN N° 82 Forecasts and Dekadal Climate Alerts for the Period 1st to 10th June 2021 st 1 June 2021 © NOCC June 2021, all rights reserved Supervision Prof. Dr. Eng. AMOUGOU Joseph Armathé, Director General, National Observatory on Climate Change (NOCC) and Lecturer in the Department of Geography at the University of Yaounde I, Cameroon. Eng. FORGHAB Patrick MBOMBA, Deputy Director General, National Observatory on Climate Change (NOCC). Production Team (NOCC) Prof. Dr. Eng. AMOUGOU Joseph Armathé, Director General, National Observatory on Climate Change (NOCC) and Lecturer in the Department of Geography at the University of Yaounde I, Cameroon. Eng. FORGHAB Patrick MBOMBA, Deputy Director General, National Observatory on Climate Change (NOCC). BATHA Romain Armand Soleil, PhD student and Technical staff, NOCC. ZOUH TEM Isabella, M.Sc. in GIS-Environment and Technical staff, NOCC. NDJELA MBEIH Gaston Evarice, M.Sc. in Economics and Environmental Management. MEYONG René Ramsès, M.Sc. in Physical Geography (Climatology/Biogeography). ANYE Victorine Ambo, Administrative staff, NOCC. ELONG Julien Aymar, M.Sc. in Business and Environmental law. I. Introduction -

Programmation De La Passation Et De L'exécution Des Marchés Publics
PROGRAMMATION DE LA PASSATION ET DE L’EXÉCUTION DES MARCHÉS PUBLICS EXERCICE 2021 JOURNAUX DE PROGRAMMATION DES MARCHÉS DES SERVICES DÉCONCENTRÉS ET DES COLLECTIVITÉS TERRITORIALES DÉCENTRALISÉES RÉGION DU LITTORAL EXERCICE 2021 SYNTHESE DES DONNEES SUR LA BASE DES INFORMATIONS RECUEILLIES Nbre de N° Désignation des MO/MOD Montant des Marchés N° page Marchés 1 Services déconcentrés Régionaux 11 476 050 000 3 2 Communauté Urbaine de Nkongsamba 143 49 894 418 496 4 3 Communauté Urbaine de Nkongsamba 1 125 000 000 16 Département du Moungo 4 Services déconcentrés départementaux 2 38 000 000 17 5 Commune de BARE BAKEM 9 312 790 000 17 6 Commune de BONALEA 24 412 000 000 17 7 Commune de DIBOMBARI 11 273 300 000 19 8 Commune de LOUM 8 186 600 000 20 9 Commune de MANJO 8 374 700 000 21 10 Commune de MBANGA 9 222 600 000 21 11 Commune de MELONG 13 293 140 184 22 12 Commune de NJOMBE PENJA 5 221 710 000 23 13 Commune d'EBONE 10 294 400 000 24 14 Commune de MOMBO 6 142 500 000 24 15 Commune de NKONGSAMBA I 11 245 833 000 25 16 Commune de NKONGSAMBA II 11 316 000 000 26 17 Commune de NKONGSAMBA III 6 278 550 000 27 TOTAL Département 133 3 612 123 184 Département du Nkam 18 Services déconcentrés départementaux 2 16 000 000 28 19 Commune de NDOBIAN 12 309 710 000 28 20 Commune de NKONDJOCK 8 377 000 000 29 21 Commune de YABASSI 21 510 500 000 29 22 Commune de YINGUI 11 241 000 000 31 TOTAL Département 54 1 454 210 000 Département de la Sanaga Maritime 23 Services déconcentrés départementaux 10 371 600 000 32 24 Commune de Dibamba 13 328 650 000 32 -

Proceedingsnord of the GENERAL CONFERENCE of LOCAL COUNCILS
REPUBLIC OF CAMEROON REPUBLIQUE DU CAMEROUN Peace - Work - Fatherland Paix - Travail - Patrie ------------------------- ------------------------- MINISTRY OF DECENTRALIZATION MINISTERE DE LA DECENTRALISATION AND LOCAL DEVELOPMENT ET DU DEVELOPPEMENT LOCAL Extrême PROCEEDINGSNord OF THE GENERAL CONFERENCE OF LOCAL COUNCILS Nord Theme: Deepening Decentralization: A New Face for Local Councils in Cameroon Adamaoua Nord-Ouest Yaounde Conference Centre, 6 and 7 February 2019 Sud- Ouest Ouest Centre Littoral Est Sud Published in July 2019 For any information on the General Conference on Local Councils - 2019 edition - or to obtain copies of this publication, please contact: Ministry of Decentralization and Local Development (MINDDEVEL) Website: www.minddevel.gov.cm Facebook: Ministère-de-la-Décentralisation-et-du-Développement-Local Twitter: @minddevelcamer.1 Reviewed by: MINDDEVEL/PRADEC-GIZ These proceedings have been published with the assistance of the German Federal Ministry for Economic Cooperation and Development (BMZ) through the Deutsche Gesellschaft für internationale Zusammenarbeit (GIZ) GmbH in the framework of the Support programme for municipal development (PROMUD). GIZ does not necessarily share the opinions expressed in this publication. The Ministry of Decentralisation and Local Development (MINDDEVEL) is fully responsible for this content. Contents Contents Foreword ..............................................................................................................................................................................5 -

Promising and Best Practices in Hiv/Aids Prevention and Care for West and Central Africa
PROMISING AND BEST PRACTICES IN HIV/AIDS PREVENTION AND CARE FOR WEST AND CENTRAL AFRICA 0 In July 2011, FHI became FHI 360. FHI 360 is a nonprofit human development organization dedicated to improving lives in lasting ways by advancing integrated, locally driven solutions. Our staff includes experts in health, education, nutrition, environment, economic development, civil society, gender, youth, research and technology – creating a unique mix of capabilities to address today’s interrelated development challenges. FHI 360 serves more than 60 countries, all 50 U.S. states and all U.S. territories. Visit us at www.fhi360.org. PROMISING AND BEST PRACTICES IN STI/HIV/AIDS PREVENTION AND CARE IN WEST AND CENTRAL AFRICA AWARE-HIV/AIDS This publication was made possible through the support of the United States President’s Emergency Plan for AIDS Relief through USAID under the Cooperation Agreement 688-A-00- 03-00066-00 with Family Health International (FHI). The opinions expressed herein are those of the authors and do not necessarily reflect the views of FHI or USAID. 1 ACKNOWLEDGEMENTS The AWARE-HIV/AIDS Project team wishes to express its sincere gratitude to the following for their cooperation and contribution: West Africa Health Organization (WAHO), National AIDS Control Councils and Programs of Benin, Burkina Faso, Cameroon, Cape Verde, Chad, Côte d’Ivoire, The Gambia, Ghana, Guinea, Guinea Bissau, Liberia, Mali, Mauritania, Niger, Nigeria, Senegal, Sierra Leone and Togo, the World Health Organization (WHO), UNAIDS and Advance Africa. We would also like to thank all those who have contributed to the success of the process for selecting and documenting promising and best practices. -

Procurement Plan
Procurement Plan Community Development Program Support Project – Phase III (Programme National de Développement Participatif – Phase III) Public Disclosure Authorized I. General 1. Bank’s approval Date of the procurement Plan [Original: February 2016]: Revision of Updated Procurement Plan, May 05, 2017 2. Date of General Procurement Notice: February 15, 2016 3. Period covered by this procurement plan: The procurement period of project covered from year January to December 2017 II. Goods and Works and non-consulting services. 1. Prior Review Threshold: Procurement Decisions subject to Prior Review by the Bank as Public Disclosure Authorized stated in Appendix 1 to the Guidelines for Procurement: Procurement Method Prior Review Threshold Comments US$ 1. ICB and LIB (Goods) Above US$ 1,500,000 All 2. NCB (Goods) None 3. ICB (Works) Above US$ 5 million All 4. NCB (Works) Above US$ 5 million 5. DC (Non-Consultant Services) none 2. Prequalification. Not applicable. Public Disclosure Authorized 3. Proposed Procedures for CDD Components (as per paragraph. 3.17 of the Guidelines: Procurement (works, goods and services) for subprojects of communes, such as small infrastructure, social infrastructure, classrooms, health centers, community halls, , minor works or rehabilitation, will be procured on the basis of community participation in accordance with the clause 3.19 of the World Bank Procurement Guidelines (Community Participation in Procurement), and the Guidelines for Simplified Procurement and Disbursement for Community-Based Investments (March 3, 1998) detailed in the specific manual or guidelines to communes approved by the bank. 4. Reference to (if any) Project Operational/Procurement Manual: Guide des Acteurs intervenant dans le processus de passation et de l’exécution des marchés communaux financés par le PNDP – Juin 2010, révisé en Janvier 2016 Public Disclosure Authorized 5. -
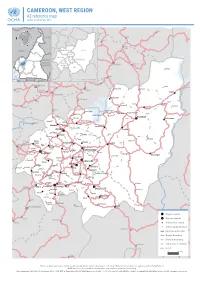
CAMEROON, WEST REGION A3 Reference Map Update of September 2018
CAMEROON, WEST REGION A3 reference map Update of September 2018 Nwa Ndu Benakuma CHAD WUM Nkor Tatum NIGERIA BAMBOUTOS NOUN FUNDONGMIFI MENOUA Elak NKOUNG-KHI CENTRAL H.-P. Njinikom AFRICAN HAUT- KUMBO Mbiame REPUBLIC -NKAM Belo NDÉ Manda Njikwa EQ. Bafut Jakiri GUINEA H.-P. : HAUTS--PLATEAUX GABON CONGO MBENGWI Babessi Nkwen Koula Koutoukpi Mabouo NDOP Andek Mankon Magba BAMENDA Bangourain Balikumbat Bali Foyet Manki II Bangambi Mahoua Batibo Santa Njimom Menfoung Koumengba Koupa Matapit Bamenyam Kouhouat Ngon Njitapon Kourom Kombou FOUMBAN Mévobo Malantouen Balepo Bamendjing Wabane Bagam Babadjou Galim Bati Bafemgha Kouoptamo Bamesso MBOUDA Koutaba Nzindong Batcham Banefo Bangang Bapi Matoufa Alou Fongo- Mancha Baleng -Tongo Bamougoum Foumbot FONTEM Bafou Nkong- Fongo- -Zem -Ndeng Penka- Bansoa BAFOUSSAM -Michel DSCHANG Momo Fotetsa Malânden Tessé Fossang Massangam Batchoum Bamendjou Fondonéra Fokoué BANDJOUN BAHAM Fombap Fomopéa Demdeng Singam Ngwatta Mokot Batié Bayangam Santchou Balé Fondanti Bandja Bangang Fokam Bamengui Mboébo Bangou Ndounko Baboate Balambo Balembo Banka Bamena Maloung Bana Melong Kekem Bapoungué BAFANG BANGANGTÉ Bankondji Batcha Mayakoue Banwa Bakou Bakong Fondjanti Bassamba Komako Koba Bazou Baré Boutcha- Fopwanga Bandounga -Fongam Magna NKONGSAMBA Ndobian Tonga Deuk Region capital Ebone Division capital Nkondjock Manjo Subdivision capital Other populated place Ndikiniméki InternationalBAF borderIA Region boundary DivisionKiiki boundary Nitoukou Subdivision boundary Road Ombessa Bokito Yingui The boundaries and names shown and the designations used on this map do not imply official endorsement or acceptance by the United Nations. NOTE: In places, the subdivision boundaries may suffer of significant inacurracy. Date of update: 23/09/2018 ● Sources: NGA, OSM, WFP ● Projection: WGS84 Web Mercator ● Scale: 1 / 650 000 (on A3) ● Availlable online on www.humanitarianresponse.info ● www.ocha.un.org. -

Ministry of Territorial Administration and Decentralization
MINISTRY OF TERRITORIAL ADMINISTRATION AND DECENTRALIZATION DEPARTMENT OF CIVIL PROTECTION Cameroon Civil Protection Report, 2008/2009 Civil defence through life-saving actions With assistance from: • United Nations Development Programme, UNDP • United Nations Children’s Fund, UNICEF • Cooperation and Cultural Action Service, French Embassy SCAC • National Hydrocarbons Company, SNH • Local Council Support Fund for Mutual Assistance, FEICOM • National Civil Engineering Laboratory, LABOGENIE Copyright © 2009 by DPC/MINATD Cameroon Civil Protection Report, 2008/2009 Theme: Civil defence through life - saving actions Initiative and coordination: Department of Civil Protection Published by: The Ministry of Territorial Administration and Decentralisation, MINATD Supported by: - United Nations Development Programme, UNDP - United Nations Children’s Fund, UNICEF - Cooperation and Cultural Action Service, French Embassy, SCAC - National Hydrocarbons Corporation, SNH - Local Council Support Fund for Mutual Assistance, FEICOM - National Civil Engineering Laboratory, LABOGENIE Consultant: Cabinet White Dove Company, BP 3774 Yaounde Editors: Guy KETCHATCHAM NGAMY ([email protected]) and Franklin Ludovic KAMTCHE Translation team leader: UBANAKO Valentine NJENDE Supervision: Minister of State, Minister of Territorial Administration and Decentralisation, MINATD This book is the sixth edition of a yearly publication on civil protection in Cameroon. All events reported cover the period between 1 December 2007 and 30 November 2008. Some of the views herein -

CONTRACTUALISATION DES MAITRES DES PARENTS DEUXIEME OPERATION AU TITRE DE L'exercice2016 Liste Nominative Des Bénéficiaires De
REPUBLIQUE DU CAMEROUN REPUBLIC OF CAMEROON Paix-Travail-Patrie Peace- Work-Fatherland MINISTERE DE ~~~~~:_ATlON ~E lASE MINISTRY OF BASIC EDUCATION SECRETARIAT GENERAL !fJ GENERAL SECRETARIAT DIRECTION DES RESSOURCES HUMAINE DEPARTMENT OF HUMAN RESOURCES COMITE DE SUIVI DES OPERATIONS DE FOLLOW UP COMMITTEE FOR THE CONTRACTUALISATION DES ENSEIGNANTS RECRUITMENT OF CONTRACT TEACHERS CONTRACTUALISATION DES MAITRES DES PARENTS DEUXIEME OPERATION AU TITRE DE L'EXERCICE 2016 Liste nominative des bénéficiaires des bons de caisse du mois de juin 2017, Région du Littoral NOMS ET PRENOMS SEXE DEPARTEMENT ARRONDISSEMENT N' MATRICULES 1 CHENDJOU ROSE ANNE Q-054844 F MOUNGO BARE-BEKEM 2 TIMMO KAMGUIA NADINE Y-054851 F MOUNGO BARE-BEKEM MOUNGO DIBOMBARI 3 ADELINE MBI MBENG V-052572 F 4 AKWI MO FOR SOLANGE G-052769 F MOUNGO DIBOMBARI MOUNGO DIBOMBARI 5 FOYANG KAMDEM BLAISE MARCEL D-053730 M 6 KENYI EMMACULATE NAHJELA U-052650 F MOUNGO DIBOMBARI 7 SOPPI VIVI AN BLANCHE M-052738 F MOUNGO DIBOMBARI 8 TCHOUBO FOUTCHOU CARINE GRECE V-052748 F MOUNGO DIBOMBARI 9 TSAPDOUM AIMEE 0-052760 F MOUNGO DIBOMBARI 10 NGULEFACPHILOMENEFONTEM 0-052666 F MOUNGO DIBOMBARI BANGU MIRANDA MOIWO F MOUNGO FIKO-BONALEA 11 X-052590 LEUGOUE TOUKO MADELEINE F MOUNGO FIKO-BONALEA 12 M-053817 MPONGO EPALLE ELIANE GWLADYS F MOUNGO FIKO-BONALEA 13 Y-053946 TIKU HERICK EYOH M MOUNGO FIKO-BONALEA 14 V-053919 15 ASSOUA NGOUO CORENTIN 5-052360 M MOUNGO LOUM 16 DJIBOUONG NGOPSEU ANNETIE MADO M-053700 F MOUNGO LOUM 17 EKA FERDINAND V-052386 M MOUNGO LOUM 18 EPOLE ASSOMO NADIA V-052388 -
How Serious Is Corruption in the Non-Wood Forest Products
Transparency and governance in non-wood forest product commodity chains, an example from Cameroon: a need to include NWFP in the FLEGT mechanism. Julius Chupezi TIEGUHONG1, Ousseynou NDOYE, Sophie GROUWELS, Armand ASSENG ZE, Juliane MASUCH, Ignace Fokou SAKAM and William MALA 1Email: [email protected] & [email protected]; Tel: +237 75622222 Fax: +237 22204811 At the World Forestry Congresses (WFC) in 2003 and 2009, good governance and efficient institutions were reiterated as necessary indicators to consider when measuring positive outcomes resulting from long-term thinking in the forest discourse at all levels. It was further stressed that without good governance and effective institutions, the scope of sustainable forest management will remain limited. This discourse dwells on concepts such as democratisation, accountability, empowerment, equity, corruption, illegality, governance and transparency. Examined in this paper are the practical applications of four of the latter interrelated concepts as they relate to the production, transportation and trade of non-wood forest products, and looking specifically at a case study on Gnetum spp, a leafy vegetable in Cameroon. Data was collected from traders on all of the financial transactions involved in accessing, transporting and selling Gnetum spp from the zone of production to the point of export over a period of one year. A total of 18368 transactions were documented during 302 journeys. 81.6% of the transactions were illegal but covered 33.5% of the total costs of all transactions (341 250 960 CFAF equivalent to US$ 780558) over the study period. There were a total of 122 locations and major reasons for making a financial transaction with only 10.7% that covered legal transactions while 65.6% covered completely illegal transactions and 23.7% both legal and illegal transactions. -

Forecasts and Dekadal Climate Alerts for the Period 11Th to 20Th May 2021
REPUBLIQUE DU CAMEROUN REPUBLIC OF CAMEROON Paix-Travail-Patrie Peace-Work-Fatherland ----------- ----------- OBSERVATOIRE NATIONAL SUR NATIONAL OBSERVATORY LES CHANGEMENTS CLIMATIQUES ON CLIMATE CHANGE ----------------- ----------------- DIRECTION GENERALE DIRECTORATE GENERAL ----------------- ----------------- ONACC www.onacc.cm; [email protected]; Tel : (+237) 693 370 504 / 654 392 529 BULLETIN N° 80 Forecasts and Dekadal Climate Alerts for the Period 11th to 20th May 2021 th 11 May 2021 © NOCC May 2021, all rights reserved Supervision Prof. Dr. Eng. AMOUGOU Joseph Armathé, Director General, National Observatory on Climate Change (NOCC) and Lecturer in the Department of Geography at the University of Yaounde I, Cameroon. Eng. FORGHAB Patrick MBOMBA, Deputy Director General, National Observatory on Climate Change (NOCC). Production Team (NOCC) Prof. Dr. Eng. AMOUGOU Joseph Armathé, Director General, National Observatory on Climate Change (NOCC) and Lecturer in the Department of Geography at the University of Yaounde I, Cameroon. Eng. FORGHAB Patrick MBOMBA, Deputy Director General, National Observatory on Climate Change (NOCC). BATHA Romain Armand Soleil, PhD student and Technical staff, NOCC. ZOUH TEM Isabella, M.Sc. in GIS-Environment and Technical staff, NOCC. NDJELA MBEIH Gaston Evarice, M.Sc. in Economics and Environmental Management. MEYONG René Ramsès, M.Sc. in Physical Geography (Climatology/Biogeography). ANYE Victorine Ambo, Administrative staff, NOCC. MEKA ZE Philemon Raissa, Administrative staff, NOCC. ELONG Julien Aymar, M.Sc. -
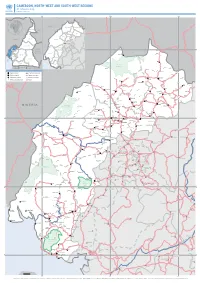
CAMEROON, NORTH-WEST and SOUTH-WEST REGIONS A1 Reference Map Update of June 2018
CAMEROON, NORTH-WEST AND SOUTH-WEST REGIONS A1 reference map Update of June 2018 9.0°E 10.0°E 11.0°E NIGERIA DONGA-MANTUNG EXTRÊME-NORD MENCHUMMENCHUM DONGA-MANTUNG FAR-NORTH CHAD BOYO BOYO BUIBUI MEZAM NGO- MOMOMOMO MEZAM NGOKETUNJIA-KETUNJIA NORTHNORD NIGERIA MANYUMANYU LEBIALEMLEBIALEM ADAMAOUAADAMAOUA NORTH- KUPE- 7.0°N NORD-OUEST-WEST CENTRAL -MUANENGUBA KUPE-MUANENGUBA Abonshie WESTOUEST AFRICAN SOUTH- SUD-OUEST REPUBLIC -WEST NDIANNDIAN Buku CENTRECENTRE Furu Awa LITTORALLITTORAL MEMEMEME EASTEST Ako SOUTHSUD FAKOFAKO Kimbi-Fungom EQ. Natianal Park GABON Akwaja GUINEA CONGO Munkep Dumbu Adere Ande Jevi Berabe Region capital International border Lus Kom Division capital Region boundary Subdivision capital Division boundary Abar NKAMBE Mfom Esu Misaje Ntong Other populated place Road Binka Iyahe Zhoa Akumaye Baworo Fonfuka Gom Ballin Nwa Yang Weh Kumfutu Mbwat Benade Lassin Ngale Ndu Benakuma Rom Benagudi Konene WUM Kuk Nkor Akwaya Sabongari Mmen Tatum Ntumbaw Modelle Aduk Ngu Nkim Assaka Ajung Befang Ntem Bu Tinta FUNDONG Mbonso Mbenkas Elak Shishong Njinikom Obang Dzeng Takamanda Mbakong KUMBO NIGERIA Mbiame Nskumba Ayi Mejang Natianal Park Tingoh Belo Ote Njikwa Oshie Babanki Ibal Tava Ngwo Tugi Bafut Jakiri Babungo Akwa Akanunku Tinachong Lip Bambui Sabga 6.0°N Mbu MBENGWI Baba I Babessi Dadi Menka Bambili NDOP 6.0°N Andek Kajifu Acha Tugui Mankon Nkwen Nyang Bamessing Bangolan Magba Ebinsi Teze Abebung BAMENDA Olorunti Kai Bangourain Akum Bambalang Oshum Balikumbat Agborkem Widikum Bali Mukoyong Bamukumbit Baliben Njimom Batibo