Maheen Adamson Transcript
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection
Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection This guide is for middle and high school students participating in AIMS Anatomy of the Human Brain and Sheep Brain Dissections. Programs will be presented by an AIMS Anatomy Specialist. In this activity students will become more familiar with the anatomical structures of the human brain by observing, studying, and examining human specimens. The primary focus is on the anatomy, function, and pathology. Those students participating in Sheep Brain Dissections will have the opportunity to dissect and compare anatomical structures. At the end of this document, you will find anatomical diagrams, vocabulary review, and pre/post tests for your students. The following topics will be covered: 1. The neurons and supporting cells of the nervous system 2. Organization of the nervous system (the central and peripheral nervous systems) 4. Protective coverings of the brain 5. Brain Anatomy, including cerebral hemispheres, cerebellum and brain stem 6. Spinal Cord Anatomy 7. Cranial and spinal nerves Objectives: The student will be able to: 1. Define the selected terms associated with the human brain and spinal cord; 2. Identify the protective structures of the brain; 3. Identify the four lobes of the brain; 4. Explain the correlation between brain surface area, structure and brain function. 5. Discuss common neurological disorders and treatments. 6. Describe the effects of drug and alcohol on the brain. 7. Correctly label a diagram of the human brain National Science Education -

Basic Brain Anatomy
Chapter 2 Basic Brain Anatomy Where this icon appears, visit The Brain http://go.jblearning.com/ManascoCWS to view the corresponding video. The average weight of an adult human brain is about 3 pounds. That is about the weight of a single small To understand how a part of the brain is disordered by cantaloupe or six grapefruits. If a human brain was damage or disease, speech-language pathologists must placed on a tray, it would look like a pretty unim- first know a few facts about the anatomy of the brain pressive mass of gray lumpy tissue (Luria, 1973). In in general and how a normal and healthy brain func- fact, for most of history the brain was thought to be tions. Readers can use the anatomy presented here as an utterly useless piece of flesh housed in the skull. a reference, review, and jumping off point to under- The Egyptians believed that the heart was the seat standing the consequences of damage to the structures of human intelligence, and as such, the brain was discussed. This chapter begins with the big picture promptly removed during mummification. In his and works down into the specifics of brain anatomy. essay On Sleep and Sleeplessness, Aristotle argued that the brain is a complex cooling mechanism for our bodies that works primarily to help cool and The Central Nervous condense water vapors rising in our bodies (Aristo- tle, republished 2011). He also established a strong System argument in this same essay for why infants should not drink wine. The basis for this argument was that The nervous system is divided into two major sec- infants already have Central nervous tions: the central nervous system and the peripheral too much moisture system The brain and nervous system. -

The Human Brain Hemisphere Controls the Left Side of the Body and the Left What Makes the Human Brain Unique Is Its Size
About the brain Cerebrum (also known as the The brain is made up of around 100 billion nerve cells - each one cerebral cortex or forebrain) is connected to another 10,000. This means that, in total, we The cerebrum is the largest part of the brain. It is split in to two have around 1,000 trillion connections in our brains. (This would ‘halves’ of roughly equal size called hemispheres. The two be written as 1,000,000,000,000,000). These are ultimately hemispheres, the left and right, are joined together by a bundle responsible for who we are. Our brains control the decisions we of nerve fibres called the corpus callosum. The right make, the way we learn, move, and how we feel. The human brain hemisphere controls the left side of the body and the left What makes the human brain unique is its size. Our brains have a hemisphere controls the right side of the body. The cerebrum is larger cerebral cortex, or cerebrum, relative to the rest of the The human brain is the centre of our nervous further divided in to four lobes: frontal, parietal, occipital, and brain than any other animal. (See the Cerebrum section of this temporal, which have different functions. system. It is the most complex organ in our fact sheet for further information.) This enables us to have abilities The frontal lobe body and is responsible for everything we do - such as complex language, problem-solving and self-control. The frontal lobe is located at the front of the brain. -

Cortical Parcellation Protocol
CORTICAL PARCELLATION PROTOCOL APRIL 5, 2010 © 2010 NEUROMORPHOMETRICS, INC. ALL RIGHTS RESERVED. PRINCIPAL AUTHORS: Jason Tourville, Ph.D. Research Assistant Professor Department of Cognitive and Neural Systems Boston University Ruth Carper, Ph.D. Assistant Research Scientist Center for Human Development University of California, San Diego Georges Salamon, M.D. Research Dept., Radiology David Geffen School of Medicine at UCLA WITH CONTRIBUTIONS FROM MANY OTHERS Neuromorphometrics, Inc. 22 Westminster Street Somerville MA, 02144-1630 Phone/Fax (617) 776-7844 neuromorphometrics.com OVERVIEW The cerebral cortex is divided into 49 macro-anatomically defined regions in each hemisphere that are of broad interest to the neuroimaging community. Region of interest (ROI) boundary definitions were derived from a number of cortical labeling methods currently in use. Protocols from the Laboratory of Neuroimaging at UCLA (LONI; Shattuck et al., 2008), the University of Iowa Mental Health Clinical Research Center (IOWA; Crespo-Facorro et al., 2000; Kim et al., 2000), the Center for Morphometric Analysis at Massachusetts General Hospital (MGH-CMA; Caviness et al., 1996), a collaboration between the Freesurfer group at MGH and Boston University School of Medicine (MGH-Desikan; Desikan et al., 2006), and UC San Diego (Carper & Courchesne, 2000; Carper & Courchesne, 2005; Carper et al., 2002) are specifically referenced in the protocol below. Methods developed at Boston University (Tourville & Guenther, 2003), Brigham and Women’s Hospital (McCarley & Shenton, 2008), Stanford (Allan Reiss lab), the University of Maryland (Buchanan et al., 2004), and the University of Toyoma (Zhou et al., 2007) were also consulted. The development of the protocol was also guided by the Ono, Kubik, and Abernathy (1990), Duvernoy (1999), and Mai, Paxinos, and Voss (Mai et al., 2008) neuroanatomical atlases. -

Anatomy of Cerebral Hemispheres Doctors Notes Notes/Extra Explanation Please View Our Editing File Before Studying This Lecture to Check for Any Changes
Color Code Important Anatomy of Cerebral Hemispheres Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of the lecture, the students should be able to: List the parts of the cerebral hemisphere (cortex, medulla, basal nuclei, lateral ventricle). Describe the subdivision of a cerebral hemisphere into lobes. List the important sulci and gyri of each lobe. Describe different types of fibers in cerebral medulla (association, projection and commissural) and give example of each type. Cerebrum Extra Corpus callosum o Largest part of the forebrain. ( makes up 2 / 3 rd weight off all brain) (recall: the forebrain gives the cerebral hemispheres and the diencephalon) o Divided into two halves, the cerebral hemispheres (right and left), which are separated Left hemisphere Right hemisphere by a deep median longitudinal fissure which lodges the falx cerebri*. o In the depth of the fissure, the hemispheres are connected by a bundle of fibers called the corpus callosum. *It is a large, crescent- shaped fold of meningeal layer of dura Median longitudinal fissure mater that descends vertically in the longitudinal fissure between the cerebral Extra Extra hemispheres Cerebrum Buried within the white matter Cerebral Hemispheres lie a number of nuclear masses The structure of cerebral hemipheres includes: (caudate, putamen, globus pallidus) collectively known as the basal ganglia. WM Deeper to the cortex, axons running to and from the cells of the cortex form an extensive mass of white matter (WM). Contains synapses (50 trillion) WM Superficial layer of grey matter, the cerebral cortex. -

SAY: Welcome to Module 1: Anatomy & Physiology of the Brain. This
12/19/2018 11:00 AM FOUNDATIONAL LEARNING SYSTEM 092892-181219 © Johnson & Johnson Servicesv Inc. 2018 All rights reserved. 1 SAY: Welcome to Module 1: Anatomy & Physiology of the Brain. This module will strengthen your understanding of basic neuroanatomy, neurovasculature, and functional roles of specific brain regions. 1 12/19/2018 11:00 AM Lesson 1: Introduction to the Brain The brain is a dense organ with various functional units. Understanding the anatomy of the brain can be aided by looking at it from different organizational layers. In this lesson, we’ll discuss the principle brain regions, layers of the brain, and lobes of the brain, as well as common terms used to orient neuroanatomical discussions. 2 SAY: The brain is a dense organ with various functional units. Understanding the anatomy of the brain can be aided by looking at it from different organizational layers. (Purves 2012/p717/para1) In this lesson, we’ll explore these organizational layers by discussing the principle brain regions, layers of the brain, and lobes of the brain. We’ll also discuss the terms used by scientists and healthcare providers to orient neuroanatomical discussions. 2 12/19/2018 11:00 AM Lesson 1: Learning Objectives • Define terms used to specify neuroanatomical locations • Recall the 4 principle regions of the brain • Identify the 3 layers of the brain and their relative location • Match each of the 4 lobes of the brain with their respective functions 3 SAY: Please take a moment to review the learning objectives for this lesson. 3 12/19/2018 11:00 AM Directional Terms Used in Anatomy 4 SAY: Specific directional terms are used when specifying the location of a structure or area of the brain. -

Sheep Brain Dissection Guide
Sheep Brain Dissection Guide DISSECTION OF THE SHEEP'S BRAIN Introduction The purpose of the sheep brain dissection is to familiarize you with the three- dimensional structure of the brain and teach you one of the great methods of studying the brain: looking at its structure. One of the great truths of studying biology is the saying that "anatomy precedes physiology". You will get sick of me saying that phrase this phrase if I teach well. What this phrase means is that how something is put together tells us much about how it works. My challenge to you with this exercise and throughout the term will be to examine a structure and think what this means about the operation of the brain. Your ideas can be as valid as anyone else's who has tackled this delightfully impossible task if you think carefully While the course will emphasize the human brain, observation and evolution indicate that there are many similarities between the sheep brain and the human brain. Even the differences are instructive and help us to learn about the brain. Being able to locate important structures in the sheep brain will be of great benefit to understanding how structures are related to each other in the human brain. If the same structure exists in both brains (and most structures are the same), they are in the same relative location. During the course of the dissection, I will point out some of the differences between brains so that you will be better able to appreciate the development of the human brain. -

Neurogenesis in the Adult Human Hippocampus
1998 Nature America Inc. • http://medicine.nature.com ARTICLES Neurogenesis in the adult human hippocampus PETER S. ERIKSSON1,4, EKATERINA PERFILIEVA1, THOMAS BJÖRK-ERIKSSON2, ANN-MARIE ALBORN1, CLAES NORDBORG3, DANIEL A. PETERSON4 & FRED H. GAGE4 Department of Clinical Neuroscience, Institute of Neurology1, Department of Oncology2, Department of Pathology3, Sahlgrenska University Hospital, 41345 Göteborg, Sweden 4Laboratory of Genetics, The Salk Institute for Biological Studies, 10010 North Torrey Pines Road, La Jolla, California 92037, USA Correspondence should be addressed to F.H.G. The genesis of new cells, including neurons, in the adult human brain has not yet been demon- strated. This study was undertaken to investigate whether neurogenesis occurs in the adult human brain, in regions previously identified as neurogenic in adult rodents and monkeys. Human brain tissue was obtained postmortem from patients who had been treated with the thymidine analog, bromodeoxyuridine (BrdU), that labels DNA during the S phase. Using im- munofluorescent labeling for BrdU and for one of the neuronal markers, NeuN, calbindin or neu- ron specific enolase (NSE), we demonstrate that new neurons, as defined by these markers, are generated from dividing progenitor cells in the dentate gyrus of adult humans. Our results further indicate that the human hippocampus retains its ability to generate neurons throughout life. Loss of neurons is thought to be irreversible in the adult human one intravenous infusion (250 mg; 2.5 mg/ml, 100 ml) of bro- brain, because dying neurons cannot be replaced. This inability modeoxyuridine (BrdU) for diagnostic purposes11. One patient to generate replacement cells is thought to be an important diagnosed with a similar type and location of cancer, but with- http://medicine.nature.com cause of neurological disease and impairment. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -
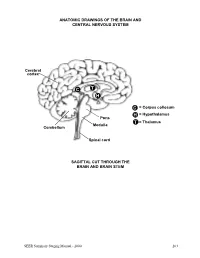
Brain and Central Nervous System
ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM Cerebral cortex C T H C = Corpus collosum H = Hypothalamus Pons T = Thalamus Medulla Cerebellum Spinal cord SAGITTAL CUT THROUGH THE BRAIN AND BRAIN STEM SEER Summary Staging Manual - 2000 263 ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM 2 1 3 4 7 5 8 6 SAGITTAL CUT THROUGH THE HUMAN HEAD WITH CEREBRUM IN PLACE The cerebrum is comprised of the: 1 Frontal lobe 2 Parietal lobe 3 Temporal lobe 4 Occipital lobe Other parts of the brain include: 5 Pons 6 Medulla (oblongata) 7 Cerebellum 8 Tentorium (cerebelli) 264 SEER Summary Staging Manual - 2000 ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM A B C D E 7 5 6 8 F SAGITTAL CUT THROUGH THE HUMAN HEAD Internal anatomy of the brain: A Inner surface of right hemisphere of cerebrum B Corpus callosum C Velum interpositum D Middle commissure E Third ventricle F Fourth ventricle Other parts of the brain (as on previous drawing): 5 Pons 6 Medulla (oblongata) 7 Cerebellum 8 Tentorium (cerebelli) SEER Summary Staging Manual - 2000 265 BRAIN AND CEREBRAL MENINGES C70.0, C71.0-C71.9 Supratentorial (S) or Infratentorial (I) C70.0 Cerebral meninges C71.0 Cerebrum ? (S) C71.1 Frontal lobe (S) C71.2 Temporal lobe (S) C71.3 Parietal lobe (S) C71.4 Occipital lobe (S) C71.5 Ventricle, NOS (S) C71.6 Cerebellum, NOS (I) C71.7 Brain stem (I) C71.8 Overlapping lesion of brain ? C71.9 Brain, NOS ? ?See Note 1. SUMMARY STAGE 1 Localized only Supratentorial tumor confined to: Cerebral hemisphere (cerebrum) or meninges of cerebral hemisphere -

Nervous System Pt 3
Write this down… Homework 2 Study Guide (Synapses) Due at the beginning of lab this week Front and back TASS M&W 1-2pm Willamette Hall 204 Thought Question… When you have one of your mandibular teeth worked on at the dentist and he gives you a shot to deaden half of your mouth, what division of the nervous system is being affected by the lidocaine? What do you think it’s mode of action is? Hint: Remember Physio-EX in lab? Is it affecting a cranial or spinal nerve? The Nervous System THE CENTRAL NERVOUS SYSTEM, T H E B R A I N Introduction Integration Memory Learning Sensation and perception Neural Tissue - Definitions White matter versus Gray matter Fiber Bundles Nerves versus Tracts Nerve Cell Bodies Nucleus versus Ganglion White and Gray Matter Central cavity surrounded by a gray matter core External white matter composed of myelinated fiber tracts Brain has additional areas of gray matter not present in spinal cord Central cavity Cortex of gray matter Migratory Inner gray pattern of matter neurons Cerebrum Outer white Cerebellum matter Gray matter Region of cerebellum Central cavity Inner gray matter Outer white matter Gray matter Brain stem Central cavity Outer white matter Inner gray matter Spinal cord Copyright © 2010 Pearson Education, Inc. Figure 12.4 Brain Similar pattern with additional areas of gray matter The Brain Conscious perception Internal regulation Average adult male 3.5 lbs Same brain mass Average adult female 3.2 lbs to body mass ratio! Brain Regions 4 Adult brain regions 1. Cerebral hemispheres (cerebrum) 2. -
Tractographic Description of the Vertical Occipital Fasciculus
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/327918090 A Connectomic Atlas of the Human Cerebrum—Chapter 16: Tractographic Description of the Vertical Occipital Fasciculus Article in Operative Neurosurgery · September 2018 DOI: 10.1093/ons/opy270 CITATIONS READS 6 114 9 authors, including: Robert G. Briggs Andrew Conner University of Southern California University of California, San Francisco 57 PUBLICATIONS 210 CITATIONS 80 PUBLICATIONS 275 CITATIONS SEE PROFILE SEE PROFILE Cordell Baker Joshua Burks University of Utah University of Miami Miller School of Medicine 40 PUBLICATIONS 162 CITATIONS 56 PUBLICATIONS 227 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Human brain networks and neurosurgery View project Connectomic Atlas of the Human Cerebrum View project All content following this page was uploaded by Michael E Sughrue on 13 October 2018. The user has requested enhancement of the downloaded file. A CONNECTOMIC ATLAS OF THE HUMAN CEREBRUM SUPPLEMENT Downloaded from https://academic.oup.com/ons/advance-article-abstract/doi/10.1093/ons/opy270/5107664 by CNS Member Access, [email protected] on 01 October 2018 A Connectomic Atlas of the Human Cerebrum—Chapter 16: Tractographic Description of the Vertical Occipital Fasciculus ∗ Robert G. Briggs, BS In this supplement, we show a comprehensive anatomic atlas of the human cerebrum Andrew K. Conner, MD∗ demonstrating all 180 distinct regions comprising the cerebral cortex. The location, Goksel Sali, MD∗ functional connectivity, and structural connectivity of these regions are outlined, and Meherzad Rahimi, BS∗ where possible a discussion is included of the functional significance of these areas.