Leptocneria Binotata
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mantodea (Insecta), with a Review of Aspects of Functional Morphology and Biology
aua o ew eaa Ramsay, G. W. 1990: Mantodea (Insecta), with a review of aspects of functional morphology and biology. Fauna of New Zealand 19, 96 pp. Editorial Advisory Group (aoimes mae o a oaioa asis MEMBERS AT DSIR PLANT PROTECTION Mou Ae eseac Cee iae ag Aucka ew eaa Ex officio ieco — M ogwo eae Sysemaics Gou — M S ugae Co-opted from within Systematics Group Dr B. A ooway Κ Cosy UIESIIES EESEAIE R. M. Emeso Eomoogy eame ico Uiesiy Caeuy ew eaa MUSEUMS EESEAIE M R. L. ama aua isoy Ui aioa Museum o iae ag Weigo ew eaa OESEAS REPRESENTATIVE J. F. awece CSIO iisio o Eomoogy GO o 1700, Caea Ciy AC 2601, Ausaia Series Editor M C ua Sysemaics Gou SI a oecio Mou Ae eseac Cee iae ag Aucka ew eaa aua o ew eaa Number 19 Maoea (Iseca wi a eiew o asecs o ucioa mooogy a ioogy G W Ramsay SI a oecio M Ae eseac Cee iae ag Aucka ew eaa emoa us wig mooogy eosigma cooaio siuaio acousic sesiiiy eece eaiou egeeaio eaio aasiism aoogy a ie Caaoguig-i-uicaio ciaio AMSAY GW Maoea (Iseca – Weigo SI uisig 199 (aua o ew eaa ISS 111-533 ; o 19 IS -77-51-1 I ie II Seies UC 59575(931 Date of publication: see cover of subsequent numbers Suggese om o ciaio amsay GW 199 Maoea (Iseca wi a eiew o asecs o ucioa mooogy a ioogy Fauna of New Zealand [no.] 19. —— Fauna o New Zealand is eae o uicaio y e Seies Eio usig comue- ase e ocessig ayou a ase ie ecoogy e Eioia Aisoy Gou a e Seies Eio ackowege e oowig co-oeaio SI UISIG awco – sueisio o oucio a isiuio M C Maews – assisace wi oucio a makeig Ms A Wig – assisace wi uiciy a isiuio MOU AE ESEAC CEE SI Miss M oy -

The Mcguire Center for Lepidoptera and Biodiversity
Supplemental Information All specimens used within this study are housed in: the McGuire Center for Lepidoptera and Biodiversity (MGCL) at the Florida Museum of Natural History, Gainesville, USA (FLMNH); the University of Maryland, College Park, USA (UMD); the Muséum national d’Histoire naturelle in Paris, France (MNHN); and the Australian National Insect Collection in Canberra, Australia (ANIC). Methods DNA extraction protocol of dried museum specimens (detailed instructions) Prior to tissue sampling, dried (pinned or papered) specimens were assigned MGCL barcodes, photographed, and their labels digitized. Abdomens were then removed using sterile forceps, cleaned with 100% ethanol between each sample, and the remaining specimens were returned to their respective trays within the MGCL collections. Abdomens were placed in 1.5 mL microcentrifuge tubes with the apex of the abdomen in the conical end of the tube. For larger abdomens, 5 mL microcentrifuge tubes or larger were utilized. A solution of proteinase K (Qiagen Cat #19133) and genomic lysis buffer (OmniPrep Genomic DNA Extraction Kit) in a 1:50 ratio was added to each abdomen containing tube, sufficient to cover the abdomen (typically either 300 µL or 500 µL) - similar to the concept used in Hundsdoerfer & Kitching (1). Ratios of 1:10 and 1:25 were utilized for low quality or rare specimens. Low quality specimens were defined as having little visible tissue inside of the abdomen, mold/fungi growth, or smell of bacterial decay. Samples were incubated overnight (12-18 hours) in a dry air oven at 56°C. Importantly, we also adjusted the ratio depending on the tissue type, i.e., increasing the ratio for particularly large or egg-containing abdomens. -
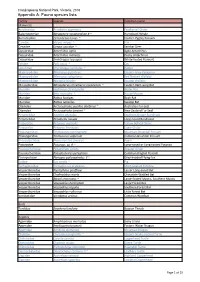
Report-VIC-Croajingolong National Park-Appendix A
Croajingolong National Park, Victoria, 2016 Appendix A: Fauna species lists Family Species Common name Mammals Acrobatidae Acrobates pygmaeus Feathertail Glider Balaenopteriae Megaptera novaeangliae # ~ Humpback Whale Burramyidae Cercartetus nanus ~ Eastern Pygmy Possum Canidae Vulpes vulpes ^ Fox Cervidae Cervus unicolor ^ Sambar Deer Dasyuridae Antechinus agilis Agile Antechinus Dasyuridae Antechinus mimetes Dusky Antechinus Dasyuridae Sminthopsis leucopus White-footed Dunnart Felidae Felis catus ^ Cat Leporidae Oryctolagus cuniculus ^ Rabbit Macropodidae Macropus giganteus Eastern Grey Kangaroo Macropodidae Macropus rufogriseus Red Necked Wallaby Macropodidae Wallabia bicolor Swamp Wallaby Miniopteridae Miniopterus schreibersii oceanensis ~ Eastern Bent-wing Bat Muridae Hydromys chrysogaster Water Rat Muridae Mus musculus ^ House Mouse Muridae Rattus fuscipes Bush Rat Muridae Rattus lutreolus Swamp Rat Otariidae Arctocephalus pusillus doriferus ~ Australian Fur-seal Otariidae Arctocephalus forsteri ~ New Zealand Fur Seal Peramelidae Isoodon obesulus Southern Brown Bandicoot Peramelidae Perameles nasuta Long-nosed Bandicoot Petauridae Petaurus australis Yellow Bellied Glider Petauridae Petaurus breviceps Sugar Glider Phalangeridae Trichosurus cunninghami Mountain Brushtail Possum Phalangeridae Trichosurus vulpecula Common Brushtail Possum Phascolarctidae Phascolarctos cinereus Koala Potoroidae Potorous sp. # ~ Long-nosed or Long-footed Potoroo Pseudocheiridae Petauroides volans Greater Glider Pseudocheiridae Pseudocheirus peregrinus -

ARTHROPODA Subphylum Hexapoda Protura, Springtails, Diplura, and Insects
NINE Phylum ARTHROPODA SUBPHYLUM HEXAPODA Protura, springtails, Diplura, and insects ROD P. MACFARLANE, PETER A. MADDISON, IAN G. ANDREW, JOCELYN A. BERRY, PETER M. JOHNS, ROBERT J. B. HOARE, MARIE-CLAUDE LARIVIÈRE, PENELOPE GREENSLADE, ROSA C. HENDERSON, COURTenaY N. SMITHERS, RicarDO L. PALMA, JOHN B. WARD, ROBERT L. C. PILGRIM, DaVID R. TOWNS, IAN McLELLAN, DAVID A. J. TEULON, TERRY R. HITCHINGS, VICTOR F. EASTOP, NICHOLAS A. MARTIN, MURRAY J. FLETCHER, MARLON A. W. STUFKENS, PAMELA J. DALE, Daniel BURCKHARDT, THOMAS R. BUCKLEY, STEVEN A. TREWICK defining feature of the Hexapoda, as the name suggests, is six legs. Also, the body comprises a head, thorax, and abdomen. The number A of abdominal segments varies, however; there are only six in the Collembola (springtails), 9–12 in the Protura, and 10 in the Diplura, whereas in all other hexapods there are strictly 11. Insects are now regarded as comprising only those hexapods with 11 abdominal segments. Whereas crustaceans are the dominant group of arthropods in the sea, hexapods prevail on land, in numbers and biomass. Altogether, the Hexapoda constitutes the most diverse group of animals – the estimated number of described species worldwide is just over 900,000, with the beetles (order Coleoptera) comprising more than a third of these. Today, the Hexapoda is considered to contain four classes – the Insecta, and the Protura, Collembola, and Diplura. The latter three classes were formerly allied with the insect orders Archaeognatha (jumping bristletails) and Thysanura (silverfish) as the insect subclass Apterygota (‘wingless’). The Apterygota is now regarded as an artificial assemblage (Bitsch & Bitsch 2000). -
Home Pre-Fire Moth Species List by Species
Species present before fire - by species Scientific Name Common Name Family Abantiades aphenges Hepialidae Abantiades hyalinatus Mustard Ghost Moth Hepialidae Abantiades labyrinthicus Hepialidae Acanthodela erythrosema Oecophoridae Acantholena siccella Oecophoridae Acatapaustus leucospila Nolidae Achyra affinitalis Cotton Web Spinner Crambidae Aeolochroma mniaria Geometridae Ageletha hemiteles Oecophoridae Aglaosoma variegata Notodontidae Agriophara discobola Depressariidae Agrotis munda Brown Cutworm Noctuidae Alapadna pauropis Erebidae Alophosoma emmelopis Erebidae Amata nigriceps Erebidae Amelora demistis Pointed Cape Moth Geometridae Amelora sp. Cape Moths Geometridae Antasia flavicapitata Geometridae Anthela acuta Common Anthelid Moth Anthelidae Anthela ferruginosa Anthelidae Anthela repleta Anthelidae Anthela sp. Anthelidae Anthela varia Variable Anthelid Anthelidae Antipterna sp. Oecophoridae Ardozyga mesochra Gelechiidae Ardozyga sp. Gelechiidae Ardozyga xuthias Gelechiidae Arhodia lasiocamparia Pink Arhodia Geometridae Arrade destituta Erebidae Arrade leucocosmalis Erebidae Asthenoptycha iriodes Tortricidae Asura lydia Erebidae Azelina biplaga Geometridae Barea codrella Oecophoridae Calathusa basicunea Nolidae Calathusa hypotherma Nolidae Capusa graodes Geometridae Capusa sp. Geometridae Carposina sp. Carposinidae Casbia farinalis Geometridae Casbia sp. Geometridae Casbia tanaoctena Geometridae Catacometes phanozona Oecophoridae Catoryctis subparallela Xyloryctidae Cernia amyclaria Geometridae Chaetolopha oxyntis Geometridae Chelepteryx -

Phylogenomics Reveals Major Diversification Rate Shifts in The
bioRxiv preprint doi: https://doi.org/10.1101/517995; this version posted January 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 Phylogenomics reveals major diversification rate shifts in the evolution of silk moths and 2 relatives 3 4 Hamilton CA1,2*, St Laurent RA1, Dexter, K1, Kitching IJ3, Breinholt JW1,4, Zwick A5, Timmermans 5 MJTN6, Barber JR7, Kawahara AY1* 6 7 Institutional Affiliations: 8 1Florida Museum of Natural History, University of Florida, Gainesville, FL 32611 USA 9 2Department of Entomology, Plant Pathology, & Nematology, University of Idaho, Moscow, ID 10 83844 USA 11 3Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, UK 12 4RAPiD Genomics, 747 SW 2nd Avenue #314, Gainesville, FL 32601. USA 13 5Australian National Insect Collection, CSIRO, Clunies Ross St, Acton, ACT 2601, Canberra, 14 Australia 15 6Department of Natural Sciences, Middlesex University, The Burroughs, London NW4 4BT, UK 16 7Department of Biological Sciences, Boise State University, Boise, ID 83725, USA 17 *Correspondence: [email protected] (CAH) or [email protected] (AYK) 18 19 20 Abstract 21 The silkmoths and their relatives (Bombycoidea) are an ecologically and taxonomically 22 diverse superfamily that includes some of the most charismatic species of all the Lepidoptera. 23 Despite displaying some of the most spectacular forms and ecological traits among insects, 24 relatively little attention has been given to understanding their evolution and the drivers of 25 their diversity. -

The New Crop Industries Handbook—Native Foods
The New Crop Industries Handbook Native foods Native Foods Book.indb 1 18/02/2008 2:05:26 PM © 2008 Rural Industries Research and Development Corporation, Canberra. All rights reserved. This handbook can be reproduced in whole or in part for studying or training purposes subject to the inclusion of an acknowledgment of the source and no commercial usage or sale. ISBN 1 74151 610 2 ISSN 1321 2656 Pub. No. 08/021 Project No. HAS-11A The New Crop Industries Handbook—Native Foods RIRDC shall not be responsible in any manner whatsoever to any person who relies, in whole or in part, on the contents of this handbook unless authorised in writing by the Managing Director of RIRDC. The handbook contains certain references to use of particular pesticides. No representation or warranty, express or implied, is made in relation to whether a particular brand of pesticide is preferable over another or whether a particular chemical product is registered by the National Registration Authority. In submitting these reports the researchers have agreed to RIRDC publishing them in edited form. RIRDC contact details Rural Industries Research and Development Corporation Level 2 15 National Circuit BARTON ACT 2600 PO Box 4776 KINGSTON ACT 2604 Tel: 02 6271 4100 Fax: 02 6271 4199 Email: [email protected] Web: www.rirdc.gov.au On-line bookshop: www.rirdc.gov.au/eshop This extract of The New Crop Industries Handbook (RIRDC Pub. No. 04/125) was printed in February 2008 Design, layout and typesetting by the RIRDC Publications Unit Printed by Union Offset Printing, Canberra Native Foods Book.indb 2 18/02/2008 2:05:26 PM Native foods Overview 1 Bush tomato 9 Lemon myrtle 16 Native citrus 21 Native pepper 31 Quandong 36 The Davidson plum 40 iii Native Foods Book.indb 3 18/02/2008 2:05:26 PM Foreword Farmers today, both those in existing businesses and new entrants, live in an environment where they by necessity have to keep an eye on new opportunities. -

LABORATORY STUDIES on the EGYPTIAN PRAYING MANTIS Miomantis Paykullii STAL, (MANTODEA: MANTIDAE) Ata, T
J. Plant Prot. and Path., Mansoura Univ., Vol. 3 (11): 1235 - 1240, 2012 LABORATORY STUDIES ON THE EGYPTIAN PRAYING MANTIS Miomantis paykullii STAL, (MANTODEA: MANTIDAE) Ata, T. E. Dep. of Plant Protection. Fac. of Agric. Al-Azhar University. Cairo. Egypt. ABSTRACT Certain biological aspects of the Egyptian praying mantis Miomantis paykullii (Stal, 1871) were studied in the laboratory. Results indicated that ootheca contained an average of 52.4 nymphs, with a mean length of 15.0 mm. The total duration period of nymphal stage required 64.1±10.54 and 54.8±6.54 days for female and male respectively. The generation period was 99 days at daily mean of temperature 25.9Cº and relative humidity percentage 45.0%. Six nymphal instars were recorded for male whereas, seven instars were found for female. The fecundity of M. paykullii through the oviposition period was 336.2±56.78 nymph per female. INTRODUCTION Miomantis paykullii (Stal, 1871). is a species of praying mantis belonging to genus Miomantis, family Mantidae, order Mantodea. Genus Miomantis has two synonym Calidomantis (Rehn 1901) and Oreomantis (Uvarov 1935), while species paykullii has four synonym savignyi (Saussure 1872), pharaonica (Saussure 1898), senegalensis (Schulthess-Rechberg 1899) and pharaonis (Kirby 1904). This species was found in different African countries, including Egypt, Ivory Coast, Ghana, Uganda, Senegal and Togo. Mantodea is a small insect order in number of species, it represented by 2452 species in 446 genera and 14 families distributed all over the world (Otte & Spearman, 2005). Mantids of Egypt form one of unique and sophisticated mantis fauna on the planet, with 59 species in 21 genera and four families (Sawaby Rabab et al. -

Integrated Pest and Disease Management Manual for Persimmon
Department of Agriculture and Fisheries INTEGRATED PEST AND DISEASE MANAGEMENT MANUAL FOR PERSIMMON Alan George, Bob Nissen, Grant Bignell, Don Hutton, Roger Broadley and David Bruun Second Edition – November 2017 Authors Alan George, Director, Asia Pacific Agricultural Consultants Pty Ltd Bob Nissen, Director, Ag-Hort International Pty Ltd Grant Bignell, Research Scientist, Department of Agriculutre and Fishereis Don Hutton Roger Broadley David Bruun, Field Operations Manager, Department of Agriculture and Fisheries We would also like to acknowledge the valuable contributions by Kent Andrews, Nic Hobbs, Geoff Patterson, Rod Dalton, Stephen Jeffers, Jeanette Wilson and many other persimmon growers. Hort Innovation and Department of Agriculture and Fisheries make no representations and expressly disclaim all warranties (to the extent permitted by law) about the accuracy, completeness, or currency of information in this Final Report. Users of this Final Report should take independent action to confirm any information in this Final Report before relying on that information in any way. Reliance on any information provided by Hort Innovation is entirely at your own risk. HIA Ltd is not responsible for, and will not be liable for, any loss, damage, claim, expense, cost (including legal costs) or other liability arising in any way (including from HIA Ltd or any other person’s negligence or otherwise) from your use or non‐use of the Final Report or from reliance on information contained in the Final Report or that HIA Ltd provides to you by any other means. This project has been funded by Horticulture Innovation Australia Limited using the Australian Persimmon industry levy and funds from the Australian Government. -

Cannibalistic Siblicide in Praying Mantis Nymphs (Miomantis Caffra)
J Ethol (2014) 32:43–51 DOI 10.1007/s10164-013-0391-z ARTICLE Cannibalistic siblicide in praying mantis nymphs (Miomantis caffra) Murray P. Fea • Margaret C. Stanley • Gregory I. Holwell Received: 6 February 2013 / Accepted: 1 November 2013 / Published online: 22 November 2013 Ó Japan Ethological Society and Springer Japan 2013 Abstract Inclusive fitness theory predicts that cannibal- Introduction ism should be more likely to arise if close relatives can be avoided, suggesting that cannibalistic species will possess Many biological traits can be understood more clearly in mechanisms for minimizing predation on kin. Juvenile the light of kin selection theory (Mock and Parker 1998), Miomantis caffra are good candidates for the possession of which demonstrates how individuals may increase their such traits because; (1) groups of siblings hatch together total fitness by aiding their relatives, due to the high pro- into the same locale, (2) they are aggressive hunters, and portion of genes shared among them (Hamilton 1964; West (3) they are strongly cannibalistic. In this study, the pos- and Gardner 2010). This may facilitate the evolution of sibility of kin recognition or avoidance in M. caffra is altruistic behavior (e.g., Nam et al. 2010). Hamilton (1964) investigated by laboratory comparison of cannibalism rates highlighted two ways in which kin selection may act, between groups of differing relatedness. In order to through kin recognition or high population viscosity. examine the likelihood of encounters between early instar Although most studies testing kin selection theory focus on siblings, the extent of dispersal away from the ootheca in the helping of kin, in regard to intraspecific aggression, theory the days following hatching is also observed. -

Foliage Insect Diversity in Dry Eucalypt Forests in Eastern Tasmania
Papers and Proceedings of the Royal Society of Tasmania, Volume 136, 2002 17 FOLIAGE INSECT DIVERSITY IN DRY EUCALYPT FORESTS IN EASTERN TASMANIA by H.J. Elliott, R. Bashford, S.J. Jarman and M.G. Neyland (with four tables, one text-figure and two appendices) ELLIOTT, H.]., BASHFORD, R., JARMAN,S.]' & NEYLAND, M.G., 2002 (3l:xii): Foliage insect diversity in dry eucalypt forests in eastern Tasmania. Papers and Proceedings ofthe Royal Society afTasmania 136: 17-34. ISSN 0080-4703. Forestry Tasmania, 79 Melville St., Hobart, Tasmania 7000, Australia. Species numbers and composition of the insect fauna occurring on trees and shrubs were studied in dry eucalypt forests in eastern Tasmania over nine years. In all, 1164 named and putative species representing 17 orders and 157 families were collected. The bulk of the species belonged to the orders Coleoptera (28%), Hymenoptera (25%), Hemiptera (18%), Lepidoptera (14%) and Diptera (10%). Of the species collected, 388 -- about one-third -- were identified at least to genus or species level. These included 21 named species not previously listed in the Tasmanian insect fauna and 90 undescribed species. A list of 22 host plants for 171 insect species was compiled from records of 132 insect species observed feeding during the study and from previous records ofinsect/host plant associations for 39 insect species found on the study plots. Most insects were feeding on eucalypts (127 insect species) and acacias (38 species). The most widely distributed and commonly collected species were several well-known pests ofeucalypts: Gonipterus scutellatus (Coleoptera: Curculionidae), Uraba lugens (Lepidoptera: N octuidae), Amorbus obscuricornis (Hemiptera: Coreidae), Chaetophyes compacta (Hemiptera: Machaerotidae) and Eriococcus coriaceous(Hemiptera: Eriococcidae). -
Moths Postfire to Feb16 2021 by Family
Species present after fire - by family Scientific Name Common Name Taxon Family Name Anthela acuta Common Anthelid Moth Anthelidae Anthela ferruginosa Anthelidae Anthela ocellata Eyespot Anthelid Moth Anthelidae Labdia chryselectra Cosmopterigidae Limnaecia sp. Cosmopterigidae Limnaecia camptosema Cosmopterigidae Macrobathra alternatella Cosmopterigidae Macrobathra astrota Cosmopterigidae Macrobathra leucopeda Cosmopterigidae Ptilomacra senex Cossidae Achyra affinitalis Cotton Web Spinner Crambidae Culladia cuneiferellus Crambidae Eudonia aphrodes Crambidae Hednota sp. Crambidae Hednota bivittella Crambidae Hednota pleniferellus Crambidae Heliothela ophideresana Crambidae Hellula hydralis cabbage centre grub Crambidae Hygraula nitens Pond moth Crambidae Metasia capnochroa Crambidae Metasia dicealis Crambidae Metasia liophaea Crambidae Musotima nitidalis Golden-brown Fern Moth Crambidae Musotima ochropteralis Australian maidenhair fern moth Crambidae Nacoleia oncophragma Crambidae Nacoleia rhoeoalis Crambidae Scoparia chiasta Crambidae Scoparia emmetropis Crambidae Scoparia exhibitalis Crambidae Scopariinae Moss-eating Crambid Snout Moths Crambidae Tipanaea patulella White Rush Moth Crambidae Agriophara confertella Depressariidae Eupselia beatella Depressariidae Eupselia carpocapsella Common Eupselia Moth Depressariidae Eutorna tricasis Depressariidae Peritropha oligodrachma Depressariidae Phylomictis sp. Depressariidae Amata nigriceps Erebidae Cyme structa Erebidae Dasypodia cymatodes Northern wattle moth Erebidae Dasypodia selenophora Southern