A First Step Towards Successful Conservation
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
Mardon Skipper Site Management Plans
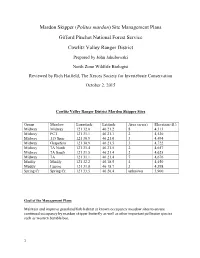
Mardon Skipper (Polites mardon) Site Management Plans Gifford Pinchot National Forest Service Cowlitz Valley Ranger District Prepared by John Jakubowski North Zone Wildlife Biologist Reviewed by Rich Hatfield, The Xerces Society for Invertebrate Conservation October 2, 2015 Cowlitz Valley Ranger District Mardon Skipper Sites Group Meadow Longitude Latitude Area (acres) Elevation (ft.) Midway Midway 121 32.0 46 21.2 8 4,313 Midway PCT 121 31.1 46 21.1 2 4,530 Midway 115 Spur 121 30.9 46 21.0 3 4,494 Midway Grapefern 121 30.9 46 21.5 3 4,722 Midway 7A North 121 31.4 46 21.5 2 4,657 Midway 7A South 121 31.5 46 21.4 2 4,625 Midway 7A 121 31.1 46 21.4 7 4,676 Muddy Muddy 121 32.2 46.18.5 4 4,450 Muddy Lupine 121 31.8 46 18.7 3 4,398 Spring Cr Spring Cr. 121 33.5 46 20.4 unknown 3,900 Goal of the Management Plans Maintain and improve grassland/forb habitat at known occupancy meadow sites to ensure continued occupancy by mardon skipper butterfly as well as other important pollinator species such as western bumble bee. 1 Introduction On the Gifford Pinchot National Forest (GPNF), mardon skippers were first detected on the Mt. Adams Ranger District (MTA) in 2000 and on Cowlitz Valley Ranger District (CVRD) in 2002. Mardon skippers are known to inhabit ten, upland dry grassy meadows on the CVRD. Portions of the meadows are mesic and are unsuitable mardon skipper habitat. -

Oviposition Selection in Montane Habitats, Biological Conservation
Biological Conservation 143 (2010) 862–872 Contents lists available at ScienceDirect Biological Conservation journal homepage: www.elsevier.com/locate/biocon Oviposition selection by a rare grass skipper Polites mardon in montane habitats: Advancing ecological understanding to develop conservation strategies Loni J. Beyer, Cheryl B. Schultz * Washington State University Vancouver, 14204 NE Salmon Creek Ave, Vancouver, WA 98686, USA article info abstract Article history: The Grass skipper subfamily (Hesperiinae) includes many at risk species across the globe. Conservation Received 31 March 2009 efforts for these skippers are hindered by insufficient information about their basic biology. Mardon skip- Received in revised form 3 November 2009 per (Polites mardon) is declining throughout its range. We surveyed mardon oviposition across nine study Accepted 25 December 2009 meadows in the Gifford Pinchot National Forest of Washington State. We conducted habitat surveys with Available online 21 January 2010 respect to oviposition (n = 269) and random (n = 270) locations, recording data on over 50 variables. Mar- don oviposited on 23 different graminoid species, yet are selective for specific graminoids within mead- Keywords: ows. Most frequent ovipositions across meadows occurred on Festuca idahoensis and Poa pratensis Butterfly (accounting for 112 of 269 total oviposition observations). Discriminant Function Analyses revealed that Graminoids Habitat preference mardon habitat was too variable to detect oviposition selection across study meadows, yet there was 2 Host selection strong selection occurring within meadows (r ranging from 0.82 to 0.99). Variables important to within Life history meadow selection were graminoid cover, height, and community; oviposition plant structure (leaf den- Management sity, height, area); insolation factors (tree abundance and canopy shading); and litter layer factors (cover Meadow and depth). -

Polites Mardon COMMON NA
U.S. FISH AND WILDLIFE SERVICE SPECIES ASSESSMENT AND LISTING PRIORITY ASSIGNMENT FORM SCIENTIFIC NAME: Polites mardon COMMON NAME: Mardon skipper LEAD REGION: Region 1 INFORMATION CURRENT AS OF: April 2007 STATUS/ACTION Species assessment ___ New candidate _X_ Continuing candidate ___ Non-petitioned _X_ Petitioned - Date petition received: 12/11/02 90-day positive - FR date: 12-month warranted but precluded - FR date: Did the petition request a reclassification of a listed species? FOR PETITIONED CANDIDATE SPECIES: a. Is listing warranted (if yes, see summary of threats below) YES b. To date, has publication of a proposal to list been precluded by other higher priority listing actions? YES c. If the answer to a. and b. is yes, provide an explanation of why the action is precluded. We find that the immediate issuance of a proposed rule and timely promulgation of a final rule for this species has been, for the preceding 12 months, and continues to be, precluded by higher priority listing actions (including candidate species with lower LPNs). During the past 12 months, almost our entire national listing budget has been consumed by work on various listing actions to comply with court orders and court- approved settlement agreements, meeting statutory deadlines for petition findings or listing determinations, emergency listing evaluations and determinations, and essential litigation-related, administrative, and program management tasks. We will continue to monitor the status of this species as new information becomes available. This review will determine if a change in status is warranted, including the need to make prompt use of emergency listing procedures. -

Management Plans for Mardon Skipper (Polites Mardon Ssp
Management Plans for mardon skipper (Polites mardon ssp. klamathensis) sites on Lily Glen and Howard Prairie Prepared by Rich Hatfield, Scott Hoffman Black, and Sarina Jepsen The Xerces Society for Invertebrate Conservation March 14, 2013 U.S.D.A. Forest Service Region 6 and U.S.D.I. Bureau of Land Management Interagency Special Status and Sensitive Species Program Table of Contents Section 1: Status and Threats ....................................................................................................... 3 Background ................................................................................................................................ 3 History and Taxonomy of Mardon Skipper in Southern Oregon ........................................... 3 Species Range, Distribution, Abundance, and Trends ............................................................ 4 Species Life History ................................................................................................................ 4 Recent Searches for Mardon Skipper in the Southern Oregon Cascades ............................... 6 Status of BLM sites in the southern Oregon Cascades ........................................................... 7 Howard Prairie and Lily Glen ................................................................................................. 8 Threats ....................................................................................................................................... 8 Small Populations .................................................................................................................. -

Community Planning and Economic Development Creating Solutions for Our Future Joshua Cummings, Director
COUNTY COMMISSIONERS John Hutchings District One Gary Edwards District Two Tye Menser District Three Community Planning and Economic Development Creating Solutions for Our Future Joshua Cummings, Director 2020 Thurston County Community Planning Field Screening Guidelines for Prairie Habitat Section 1 - 1.1 Purpose Under the development of the Habitat Conservation Plan (HCP), it is the long-term goal of Thurston County to conserve and restore large, intact areas of prairie habitat in addition to smaller tracts of land within 1/2 mi of larger prairies (Chapter 24.25.065 Thurston County Code (TCC)). While the screening process described in this protocol focuses on the detection of diagnostic prairie flora listed in the CAO, the overall intention for prairie conservation under the CAO and pending the HCP is to protect a much broader range of prairie butterflies, birds and mammals, and habitat. South Puget Sound Prairie ecosystems support a wide range of rare flora and fauna, some of which are listed under federal or state protection, including butterflies which are considered Species of Conservation Concern (SCC) or Greatest Conservation Need (SGCN). Particular attention is given to the protection of federally listed and imperiled butterfly species in Thurston County, such as the Taylor’s checkerspot (TCB, Euphydryas editha taylori), Puget blue (Icaria icarioides blackmorei), hoary elfin (Callophrys polios), Oregon branded skipper (Hesperia Colorado oregonia), Mardon skipper (Polites mardon), and valley silverspot (Speyeria zerene) butterflies, and the plant species known to serve as host and nectar plants for these butterflies. Other federally listed and candidate prairie species include the streaked horned lark (Eromophila alpestris strigata), Mazama pocket gopher (Thomomys mazama), and the Oregon vesper sparrow (Pooecetes gramineus). -

Species Fact Sheet Mardon Skipper Polites Mardon Current and Historical Status
Species Fact Sheet Mardon Skipper Polites mardon Photo Credit: Holmes, Conboy NWR STATUS: CANDIDATE Mardon skipper potentially occurs in these Washington counties: Skamania, Yakima, Klickitat, Pierce, and Thurston (Map may reflect historical as well as recent sightings) The Mardon skipper butterfly, Polites mardon, was designated a federal candidate species in 1999. Current and Historical Status The historical status of the Mardon skipper is not precisely known. Abundance estimates and distribution for this species prior to 1980 are nonexistent. This species most likely declined with the decline of early seral grassland habitat. Post 1980, mardon skippers were historically collected from four widely separated locations: the south Puget Sound region of Washington, the southern Washington Cascades, the Siskiyou Mountains of southern Oregon, and coastal northern California. Prior to 1980, very little information was known about Mardon skippers. It was thought to be endemic to Washington State only. Interest in the species has led to additional surveys and improved information on the species distribution. By 2000, this species was known to occur at more than 130 sites located in south Puget Sound and the Mt. Adams area in Washington; the Siskiyou Mountains in southern Oregon; and Del Norte County, California. All populations are relatively small with few populations supporting more than 50 individuals. In Washington, this species has been observed and is regularly surveyed on south Puget Sound prairies and on grassland openings in a forest matrix in the southern Cascades of Washington. As recently as 2009, only 2 populations were detected on south Puget Sound prairies, one at Scatter Creek Wildlife Area in Thurston County and one on the Artillery Impact Area at Fort Lewis, Pierce County. -

Management Plans for All Southern Oregon Cascade Mardon Skipper
Management Plans for all Southern Oregon Cascade Mardon Skipper (Polites mardon) sites on the Bureau of Land Management’s Hunter Creek Area of Critical Environmental Concern (ACEC) Prepared by Rich Hatfield, Scott Hoffman Black, and Sarina Jepsen, The Xerces Society for Invertebrate Conservation March 14, 2013 U.S.D.A. Forest Service Region 6 and U.S.D.I. Bureau of Land Management Interagency Special Status and Sensitive Species Program 1 Table of Contents Section 1: Status and Threats ....................................................................................................... 3 Background ................................................................................................................................ 3 History and Taxonomy of Mardon skipper in Southern Oregon ....................................... 3 Species Life History ............................................................................................................... 4 Recent Searches for Mardon Skipper. .................................................................................. 6 Status on the Hunter Creek ACEC ........................................................................................ 7 Threats ....................................................................................................................................... 8 Small Populations .................................................................................................................. 8 Climate Change ..................................................................................................................... -

Captive Rearing the Endangered Mardon Skipper
Captive Rearing the Endangered Mardon Skipper (Polites mardon) and Taylor’s Checkerspot (Euphydryas editha taylori) Butterflies: Initial Results (Lepidoptera, Nymphalidae) DANIEL N. GROSBOLL The Nature Conservancy of Washington, 120 East Union Avenue, Suite 215, Olympia, WA 98501, U.S.A., email [email protected] Abstract: Many of the butterfly species or subspecies that are largely restricted to lowland grasslands in the Willamette Valley-Puget Trough-Georgia Basin Ecoregion are known to be rare and declining. The mardon skipper (Polites mardon) is listed as Endangered in Washington. The Taylor’s checkerspot (Euphydryas editha taylori) is a Species of Concern in Washington, and is red-listed and probably extirpated in Canada. Captive rearing and reintroduction, in conjunction with habitat restoration and enhancement, are essential components in the recovery of these butterflies. Initial results from captive rearing of the Taylor’s checkerspot and mardon skipper are promising. Survival to diapause was over 80% for Taylor’s checkerspot larvae that were reared from eggs laid by captive females. The larvae were fed two different known host plants, Plantago lanceolata and Castilleja hispida, prior to diapause. Both hosts were readily accepted, and prediapause weight did not differ between larvae fed the different hosts. Initial results from diapause survival for the Taylor’s checkerspot indicate that it can survive diapause in captivity. Stimulation of egg laying, asynchrony of larval development, and pupal eclosion timing have proven to be difficulties in mardon skipper captive rearing. Key Words: mardon skipper, Polites mardon, Taylor’s checkerspot, Euphydryas editha taylori, captive rearing, reintroduction, host plant, endangered butterflies, Willamette Valley-Puget Trough-Georgia Basin Ecoregion, Washington Introduction Throughout the Willamette Valley-Puget Trough-Georgia Basin Ecoregion, grasslands with a largely native plant community have declined in extent to about 2% of the area they occupied in 1850 (Crawford and Hall 1997). -

Butterflies and Moths of Pacific Northwest Forests and Woodlands: Rare, Endangered, and Management- Sensitive Species
he Forest Health Technology Enterprise Team (FHTET) was created in 1995 by the Deputy Chief for State and Private TForestry, USDA Forest Service, to develop and deliver technologies to protect and improve the health of American forests. This book was published by FHTET as part of the technology transfer series. http://www.fs.fed.us/foresthealth/technology/ United States Depart- US Forest US Forest Service ment of Agriculture Service Forest Health Technology Enterprise Team Cover design Chuck Benedict. Photo, Taylor’s Checkerspot, Euphydryas editha taylori. Photo by Dana Ross. See page 38. For copies of this publication, contact: Dr. Jeffrey C. Miller Richard Reardon Oregon State University FHTET, USDA Forest Service Department of Rangeland Ecology 180 Canfield Street and Management Morgantown, WV 26505 202 Strand Agriculture Hall 304-285-1566 Corvallis, Washington, USA [email protected] 97331-2218 FAX 541-737-0504 Phone 541-737-5508 [email protected] The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, or marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program informa- tion (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at 202-720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326-W, Whitten Building, 1400 Inde- pendence Avenue, SW, Washington, D.C. 20250-9410 or call 202-720-5964 (voice and TDD). -

Summary of Coon Mountain, Six Rivers
CONTROLLED BURNING AND MARDON SKIPPER: SUMMARY OF MARDON SKIPPER COON MOUNTAIN BURN SITE OCCUPANCY STUDY 2009-2013. FINAL REPORT TO THE U.S. FOREST SERVICE, OREGON ZOO, AND U.S. FISH AND WILDLIFE SERVICE P H O T O G R A P H B Y C A N D A C E F ALLON SCOTT HOFFMAN BLACK, XERCES SOCIETY EXECUTIVE DIRECTOR CANDACE FALLON, XERCES SOCIETY CONSERVATION BIOLOGIST RICH HATFIELD, XERCES SOCIETY CONSERVATION BIOLOGIST CELESTE MAZZACANO, XERCES SOCIETY STAFF SCIENTIST DECEMBER 2013 Final report to the U.S. Forest Service, the Oregon Zoo, and the U.S. Fish & Wildlife Service. Summary of Mardon Skipper Coon Mountain Burn Site Occupancy Study 2009 - 2013, Xerces Society, December 2013. Page 1 CONTENTS Abstract ......................................................................................................................................................................... 3 Introduction ................................................................................................................................................................... 3 History of Mardon Skipper in California ........................................................................................................................ 4 Coon Mountain Burn Study ........................................................................................................................................... 5 Background ............................................................................................................................................................ 5 Survey -
WDFW Status Report for the Mardon Skipper
STATE OF WASHINGTON October 1999 WashingtonWashington StateState StatusStatus ReportReport forfor thethe MardonMardon SkipperSkipper by Ann Potter, John Fleckenstein, Scott Richardson and Dave Hays WWashingtonashington Department of FISH AND WILDLIFE WWildlifeildlife Management Program WDFW 631 Washington State Status Report for the Mardon Skipper by Ann Potter Wildlife Diversity Division Washington Department of Fish and Wildlife Olympia, Washington John Fleckenstein Washington Natural Heritage Program Washington Department of Natural Resources Olympia, Washington Scott Richardson Wildlife Diversity Division Washington Department of Fish and Wildlife Olympia, Washington David Hays Wildlife Diversity Division Washington Department of Fish and Wildlife Olympia, Washington October 1999 The Washington Department of Fish and Wildlife maintains a list of endangered, threatened and sensitive species (Washington Administrative Codes 232-12-014 and 232-12-011, Appendix B). In 1990, the Washington Fish and Wildlife Commission adopted listing procedures developed by a group of citizens, interest groups, and state and federal agencies (Washington Administrative Code 232-12-297, Appendix B). The procedures include how species listing will be initiated, criteria for listing and delisting, public review and recovery and management of listed species. The first step in the process is to develop a preliminary species status report. The report includes a review of information relevant to the species’ status in Washington and addresses factors affecting -
Larval Host Plant Selection and Daily Behavior of Poweshiek Skipperling
Larval Host plant Selection and Daily Behavior ofPoweshiek Skipperling (Oarismapoweshiek) in Michigan Henry Pointon Advisors: David Cuthrell Lead Zoologist Michigan Natural Features Inventory Lansing, MI Michael Monfils Avian Ecologist Michigan Natural Features Inventory Lansing, MI Ann M. Fraser Associate Professor ofBiology Kalamazoo College Kalamazoo, MI A paper submitted in partial fulfillment ofthe requirements for the degree ofBachelor ofArts at Kalamazoo College Fall 2015 Acknowledgements Special thanks to my advisors, Dave Cuthrell and Mike Monfils, for their guidance and assistance throughout the SIP process. Their help was greatly appreciated in all aspects ofseeing this project through to the end. The contributions ofthe research team from MNFI helped make this particular project possible in the middle ofa very busy field season. Their assistance helped make this project possible. This thesis would also not have been possible without the peer review group and faculty advisor: Ann Fraser, Shadi Larson, Olivia Nalugya, Charles Edick, and Sarah Woods. Table ofContents Acknowledgements i Table ofContents ii List ofFigures iii Abstract 1 Introduction 2 Methods and Materials 11 Results 15 Discussion 17 Literature Cited 19 Figures Figure 1 A (O. poweshiek ventral & dorsal view) 6 Figure 2 (O. poweshiek larva with US Penny for scale) 7 Figure 3 (M richardsonis) 8 Figure 4 (Photo ofthe prairie fen this study was conducted in) 11 Figure 5 (Satellite image ofstudy site) 13 Figure 6 (Graphical proportions ofactivity) 16 JP*\ in Abstract: Conservation efforts depend greatly on a working knowledge ofthe species of concern. Little is known about the basic biology ofmany endangered species regarding their life history information. This is true for the Poweshiek skipperling butterfly (Oarisma poweshiek), whose larval host plant is not yet known for Michigan populations.