Captive Rearing the Endangered Mardon Skipper
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Butterflies of the Wesleyan Campus
BUTTERFLIES OF THE WESLEYAN CAMPUS SWALLOWTAILS Hairstreaks (Subfamily - Theclinae) (Family PAPILIONIDAE) Great Purple Hairstreak - Atlides halesus Coral Hairstreak - Satyrium titus True Swallowtails Banded Hairstreak - Satyrium calanus (Subfamily - Papilioninae) Striped Hairstreak - Satyrium liparops Pipevine Swallowtail - Battus philenor Henry’s Elfin - Callophrys henrici Zebra Swallowtail - Eurytides marcellus Eastern Pine Elfin - Callophrys niphon Black Swallowtail - Papilio polyxenes Juniper Hairstreak - Callophrys gryneus Giant Swallowtail - Papilio cresphontes White M Hairstreak - Parrhasius m-album Eastern Tiger Swallowtail - Papilio glaucus Gray Hairstreak - Strymon melinus Spicebush Swallowtail - Papilio troilus Red-banded Hairstreak - Calycopis cecrops Palamedes Swallowtail - Papilio palamedes Blues (Subfamily - Polommatinae) Ceraunus Blue - Hemiargus ceraunus Eastern-Tailed Blue - Everes comyntas WHITES AND SULPHURS Spring Azure - Celastrina ladon (Family PIERIDAE) Whites (Subfamily - Pierinae) BRUSHFOOTS Cabbage White - Pieris rapae (Family NYMPHALIDAE) Falcate Orangetip - Anthocharis midea Snouts (Subfamily - Libytheinae) American Snout - Libytheana carinenta Sulphurs and Yellows (Subfamily - Coliadinae) Clouded Sulphur - Colias philodice Heliconians and Fritillaries Orange Sulphur - Colias eurytheme (Subfamily - Heliconiinae) Southern Dogface - Colias cesonia Gulf Fritillary - Agraulis vanillae Cloudless Sulphur - Phoebis sennae Zebra Heliconian - Heliconius charithonia Barred Yellow - Eurema daira Variegated Fritillary -

Superior National Forest
Admirals & Relatives Subfamily Limenitidinae Skippers Family Hesperiidae £ Viceroy Limenitis archippus Spread-wing Skippers Subfamily Pyrginae £ Silver-spotted Skipper Epargyreus clarus £ Dreamy Duskywing Erynnis icelus £ Juvenal’s Duskywing Erynnis juvenalis £ Northern Cloudywing Thorybes pylades Butterflies of the £ White Admiral Limenitis arthemis arthemis Superior Satyrs Subfamily Satyrinae National Forest £ Common Wood-nymph Cercyonis pegala £ Common Ringlet Coenonympha tullia £ Northern Pearly-eye Enodia anthedon Skipperlings Subfamily Heteropterinae £ Arctic Skipper Carterocephalus palaemon £ Mancinus Alpine Erebia disa mancinus R9SS £ Red-disked Alpine Erebia discoidalis R9SS £ Little Wood-satyr Megisto cymela Grass-Skippers Subfamily Hesperiinae £ Pepper & Salt Skipper Amblyscirtes hegon £ Macoun’s Arctic Oeneis macounii £ Common Roadside-Skipper Amblyscirtes vialis £ Jutta Arctic Oeneis jutta (R9SS) £ Least Skipper Ancyloxypha numitor Northern Crescent £ Eyed Brown Satyrodes eurydice £ Dun Skipper Euphyes vestris Phyciodes selenis £ Common Branded Skipper Hesperia comma £ Indian Skipper Hesperia sassacus Monarchs Subfamily Danainae £ Hobomok Skipper Poanes hobomok £ Monarch Danaus plexippus £ Long Dash Polites mystic £ Peck’s Skipper Polites peckius £ Tawny-edged Skipper Polites themistocles £ European Skipper Thymelicus lineola LINKS: http://www.naba.org/ The U.S. Department of Agriculture (USDA) prohibits discrimination http://www.butterfliesandmoths.org/ in all its programs and activities on the basis of race, color, national -
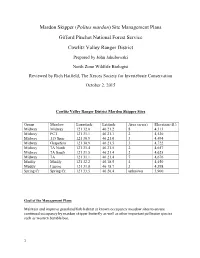
Mardon Skipper Site Management Plans
Mardon Skipper (Polites mardon) Site Management Plans Gifford Pinchot National Forest Service Cowlitz Valley Ranger District Prepared by John Jakubowski North Zone Wildlife Biologist Reviewed by Rich Hatfield, The Xerces Society for Invertebrate Conservation October 2, 2015 Cowlitz Valley Ranger District Mardon Skipper Sites Group Meadow Longitude Latitude Area (acres) Elevation (ft.) Midway Midway 121 32.0 46 21.2 8 4,313 Midway PCT 121 31.1 46 21.1 2 4,530 Midway 115 Spur 121 30.9 46 21.0 3 4,494 Midway Grapefern 121 30.9 46 21.5 3 4,722 Midway 7A North 121 31.4 46 21.5 2 4,657 Midway 7A South 121 31.5 46 21.4 2 4,625 Midway 7A 121 31.1 46 21.4 7 4,676 Muddy Muddy 121 32.2 46.18.5 4 4,450 Muddy Lupine 121 31.8 46 18.7 3 4,398 Spring Cr Spring Cr. 121 33.5 46 20.4 unknown 3,900 Goal of the Management Plans Maintain and improve grassland/forb habitat at known occupancy meadow sites to ensure continued occupancy by mardon skipper butterfly as well as other important pollinator species such as western bumble bee. 1 Introduction On the Gifford Pinchot National Forest (GPNF), mardon skippers were first detected on the Mt. Adams Ranger District (MTA) in 2000 and on Cowlitz Valley Ranger District (CVRD) in 2002. Mardon skippers are known to inhabit ten, upland dry grassy meadows on the CVRD. Portions of the meadows are mesic and are unsuitable mardon skipper habitat. -

Determinants of Successful Arthropod Eradication Programs
Biol Invasions (2014) 16:401–414 DOI 10.1007/s10530-013-0529-5 ORIGINAL PAPER Determinants of successful arthropod eradication programs Patrick C. Tobin • John M. Kean • David Maxwell Suckling • Deborah G. McCullough • Daniel A. Herms • Lloyd D. Stringer Received: 17 December 2012 / Accepted: 25 July 2013 / Published online: 27 August 2013 Ó Springer Science+Business Media Dordrecht (outside the USA) 2013 Abstract Despite substantial increases in public target species, method of detection, and the primary awareness and biosecurity systems, introductions of feeding guild of the target species. The outcome of non-native arthropods remain an unwelcomed conse- eradication efforts was not determined by program quence of escalating rates of international trade and costs, which were largely driven by the size of the travel. Detection of an established but unwanted non- infestation. The availability of taxon-specific control native organism can elicit a range of responses, tools appeared to increase the probability of eradica- including implementation of an eradication program. tion success. We believe GERDA, as an online Previous studies have reviewed the concept of erad- database, provides an objective repository of infor- ication, but these efforts were largely descriptive and mation that will play an invaluable role when future focused on selected case studies. We developed a eradication efforts are considered. Global Eradication and Response DAtabase (‘‘GER- DA’’) to facilitate an analysis of arthropod eradication Keywords Detection Á Eradication Á Invasive programs and determine the factors that influence species management Á Non-native pests eradication success and failure. We compiled data from 672 arthropod eradication programs targeting 130 non-native arthropod species implemented in 91 Introduction countries between 1890 and 2010. -

Spineless Spineless Rachael Kemp and Jonathan E
Spineless Status and trends of the world’s invertebrates Edited by Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Spineless Spineless Status and trends of the world’s invertebrates of the world’s Status and trends Spineless Status and trends of the world’s invertebrates Edited by Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Disclaimer The designation of the geographic entities in this report, and the presentation of the material, do not imply the expressions of any opinion on the part of ZSL, IUCN or Wildscreen concerning the legal status of any country, territory, area, or its authorities, or concerning the delimitation of its frontiers or boundaries. Citation Collen B, Böhm M, Kemp R & Baillie JEM (2012) Spineless: status and trends of the world’s invertebrates. Zoological Society of London, United Kingdom ISBN 978-0-900881-68-8 Spineless: status and trends of the world’s invertebrates (paperback) 978-0-900881-70-1 Spineless: status and trends of the world’s invertebrates (online version) Editors Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Zoological Society of London Founded in 1826, the Zoological Society of London (ZSL) is an international scientifi c, conservation and educational charity: our key role is the conservation of animals and their habitats. www.zsl.org International Union for Conservation of Nature International Union for Conservation of Nature (IUCN) helps the world fi nd pragmatic solutions to our most pressing environment and development challenges. www.iucn.org Wildscreen Wildscreen is a UK-based charity, whose mission is to use the power of wildlife imagery to inspire the global community to discover, value and protect the natural world. -

Butterflies Swan Island
Checkerspots & Crescents Inornate Ringlet ↓ Coenonympha tullia The Ringlet has a Harris’s Checkerspot Chlosyne harrisii bouncy, irregular Pearl Crescent ↓ Phyciodes tharos flight pattern. Abundant in open Crescents fly from fields from late May May to October. to early October. Especially common Common Wood Nymph ↓ Cercyonis pegala on dirt roads. Pearl and Northern hard The Common to differentiate. Wood Nymph is Northern Pearl Crescent Phyciodes cocyta very abundant. Flies from July to Eastern Comma Polygonia comma Sept. along wood Mourning Cloak Nymphalis antiopa edges and fields. Red Admiral Vanessa atalanta ______________________________________ Butterflies American Lady ↓ Vanessa virginiensis ______________________________________ of ______________________________________ American Lady found on dirt Swan Island roads and open Perkins TWP, Maine patches in fields. Steve Powell Wildlife Flight period is April to October. Management Area White Admiral Limenitis arthemis Viceroy Limenitis archippus ______________________________________ ______________________________________ Satyrs Great Spangled Fritillary Northern Pearly-Eye Enodia anthedon Eyed Brown ↓ Satyrodes eurydice Eyed Brown found in wet, wooded Swan Island – Explore with us! www.maine.gov/swanisland edges. Has erratic Maine Butterfly Survey flight pattern. Flies Checklist and brochure created by Robert E. mbs.umf.maine.edu from late June to Gobeil and Rose Marie F. Gobeil in cooperation with the Maine Department of Inland Fisheries Photos of Maine Butterflies early August. www.mainebutterflies.com and Wildlife (MDIFW). Photos by Rose Marie F. Gobeil Little Wood Satyr Megisto cymela ______________________________________ SECOND PRINTING: APRIL 2019 Skippers Pepper & Salt Skipper Amblyscirtes hegon Banded Hairstreak ↓ Satyrium calanus Swallowtails Banded Hairstreak: Silver-spotted Skipper ↓Epargyreus clarus uncommon, found Silver-spotted Black Swallowtail Papilio polyxenes in open glades in Skipper is large Canadian Tiger Swallowtail ↓ Papilio canadensis wooded areas. -

Oviposition Selection in Montane Habitats, Biological Conservation
Biological Conservation 143 (2010) 862–872 Contents lists available at ScienceDirect Biological Conservation journal homepage: www.elsevier.com/locate/biocon Oviposition selection by a rare grass skipper Polites mardon in montane habitats: Advancing ecological understanding to develop conservation strategies Loni J. Beyer, Cheryl B. Schultz * Washington State University Vancouver, 14204 NE Salmon Creek Ave, Vancouver, WA 98686, USA article info abstract Article history: The Grass skipper subfamily (Hesperiinae) includes many at risk species across the globe. Conservation Received 31 March 2009 efforts for these skippers are hindered by insufficient information about their basic biology. Mardon skip- Received in revised form 3 November 2009 per (Polites mardon) is declining throughout its range. We surveyed mardon oviposition across nine study Accepted 25 December 2009 meadows in the Gifford Pinchot National Forest of Washington State. We conducted habitat surveys with Available online 21 January 2010 respect to oviposition (n = 269) and random (n = 270) locations, recording data on over 50 variables. Mar- don oviposited on 23 different graminoid species, yet are selective for specific graminoids within mead- Keywords: ows. Most frequent ovipositions across meadows occurred on Festuca idahoensis and Poa pratensis Butterfly (accounting for 112 of 269 total oviposition observations). Discriminant Function Analyses revealed that Graminoids Habitat preference mardon habitat was too variable to detect oviposition selection across study meadows, yet there was 2 Host selection strong selection occurring within meadows (r ranging from 0.82 to 0.99). Variables important to within Life history meadow selection were graminoid cover, height, and community; oviposition plant structure (leaf den- Management sity, height, area); insolation factors (tree abundance and canopy shading); and litter layer factors (cover Meadow and depth). -

Winneshiek County Butterflies
A must-buy book for the butterfly enthusiast is _____Northern Broken-dash U Jn-Jl _____Coral Hairstreak C Jn-Jl The Butterflies of Iowa by Dennis W. Schlicht, John Wallengrenia egeremet Satyrium titus C. Downy and Jeffrey C. Nekola; published by the _____Little Glassywing U Jn-Jl _____Acadian Hairstreak R Jn-Jl University of Iowa Press in 2007. Pompeius verna Satyrium acadica According toThe Butterflies of Iowa, the _____Delaware Skipper C Jn-Ag _____Hickory Hairstreak R Jl following butterflies have been collected at least Anatrytone logan Satyrium caryaevorum once in Winneshiek County since records were kept. _____Hobomok Skipper C M-Jn _____Edward’s Hairstreak R Jn-Jl Poanes hobomok Satyrium edwardsii Common Name Status Flight _____Black Dash U Jn-Ag _____Banded Hairstreak U Jn-Jl Scientific Name Euphyes conspicua Satyrium calanus _____Silver Spotted Skipper C M-S _____Pipevine Swallowtail R Ag _____Gray Hairstreak U M-O Epargyreus clarus Battus philenor Strymon melinus _____Southern Cloudywing U M-Ag _____Black Swallowtail C Ap-S _____Eastern Tailed-blue A Ap-O Thorybes bathyllus Papilio polyxenes Everes comyntas _____Northern Cloudywing C Jn-Jl _____Eastern Tiger Swallowtail C Ap-S _____Summer Azure A M-S Thorybes pylades Papilio glaucus Celastrina neglecta _____Sleepy Duskywing R M _____Spicebush Swallowtail R Jn-Jl _____Reakirt’s Blue R Jn-Ag Erynnis brizo Papilio troilus Echinargus isola _____Juvenal’s Duskywing U Ap-Jn _____Giant Swallowtail U M-S _____American Snout R Jn-O Erynnis juvenalis Papilio cresphontes Libytheana carinenta -

Polites Mardon COMMON NA
U.S. FISH AND WILDLIFE SERVICE SPECIES ASSESSMENT AND LISTING PRIORITY ASSIGNMENT FORM SCIENTIFIC NAME: Polites mardon COMMON NAME: Mardon skipper LEAD REGION: Region 1 INFORMATION CURRENT AS OF: April 2007 STATUS/ACTION Species assessment ___ New candidate _X_ Continuing candidate ___ Non-petitioned _X_ Petitioned - Date petition received: 12/11/02 90-day positive - FR date: 12-month warranted but precluded - FR date: Did the petition request a reclassification of a listed species? FOR PETITIONED CANDIDATE SPECIES: a. Is listing warranted (if yes, see summary of threats below) YES b. To date, has publication of a proposal to list been precluded by other higher priority listing actions? YES c. If the answer to a. and b. is yes, provide an explanation of why the action is precluded. We find that the immediate issuance of a proposed rule and timely promulgation of a final rule for this species has been, for the preceding 12 months, and continues to be, precluded by higher priority listing actions (including candidate species with lower LPNs). During the past 12 months, almost our entire national listing budget has been consumed by work on various listing actions to comply with court orders and court- approved settlement agreements, meeting statutory deadlines for petition findings or listing determinations, emergency listing evaluations and determinations, and essential litigation-related, administrative, and program management tasks. We will continue to monitor the status of this species as new information becomes available. This review will determine if a change in status is warranted, including the need to make prompt use of emergency listing procedures. -

Papilio (New Series) # 28 2020 Issn 2372-9449
PAPILIO (NEW SERIES) # 28 2020 ISSN 2372-9449 BUTTERFLIES OF THE SOUTHERN ROCKY MOUNTAINS AREA, AND THEIR NATURAL HISTORY AND BEHAVIOR: PHOTOS OF MOSTLY EGGS LARVAE PUPAE. PART I HESPERIIDAE James A. Scott These four issues of Papilio (New Series) are photos for my book “Butterflies of the Southern Rocky Mts. Area, and their Natural History and Behavior”, showing some adults but mostly early stages (eggs, 1st-stage, mature larvae, and pupae) of as many of the species as possible, primarily from the Southern Rockies area (I added a few other interesting species that do not occur in the area). They have been cropped and downsized to illustrate just the butterflies and conserve kilobytes, rather than serve as artistic images. They are arranged by evolutionary relationship, as in the book text. Localities of photos are Colorado especially the Denver/Front Range area, unless noted. Most were taken by J. Scott, some by others (including Elton Woodbury, Steve Spomer, Frank Fee, Jim Troubridge, others listed by Scott 1986a). Abbreviations: M=male, F=female, A=adult (difficult to determine sex), E=egg, L=larva (L1=1st- stage, L2=2nd-stage, L3=3rd-stage, L4=4th-stage (usually the mature stage in Lycaeninae), L5=5th-stage usually mature in most butterflies, L6=6th stage mature in Argynnis), P=pupa Hesperiidae, Eudaminae 1 Epargyreus clarus M afternoon rest, E, L1, L5, L5, L5 prepupa, P, P Cecropterus “Thorybes” pylades pylades M, E, L1, ~L3, L5, L5, P Cecropterus “Thorybes” diversus Del Norte Co. Calif. E, L1, L5, L5, P, P Hesperiidae, Pyrginae, -

Management Plans for Mardon Skipper (Polites Mardon Ssp
Management Plans for mardon skipper (Polites mardon ssp. klamathensis) sites on Lily Glen and Howard Prairie Prepared by Rich Hatfield, Scott Hoffman Black, and Sarina Jepsen The Xerces Society for Invertebrate Conservation March 14, 2013 U.S.D.A. Forest Service Region 6 and U.S.D.I. Bureau of Land Management Interagency Special Status and Sensitive Species Program Table of Contents Section 1: Status and Threats ....................................................................................................... 3 Background ................................................................................................................................ 3 History and Taxonomy of Mardon Skipper in Southern Oregon ........................................... 3 Species Range, Distribution, Abundance, and Trends ............................................................ 4 Species Life History ................................................................................................................ 4 Recent Searches for Mardon Skipper in the Southern Oregon Cascades ............................... 6 Status of BLM sites in the southern Oregon Cascades ........................................................... 7 Howard Prairie and Lily Glen ................................................................................................. 8 Threats ....................................................................................................................................... 8 Small Populations .................................................................................................................. -
![A Study of the Life Cycle of the Chequered Skipper Butterfly Carterocephalus Palaemon (Pallas) [Online]](https://docslib.b-cdn.net/cover/8086/a-study-of-the-life-cycle-of-the-chequered-skipper-butterfly-carterocephalus-palaemon-pallas-online-1988086.webp)
A Study of the Life Cycle of the Chequered Skipper Butterfly Carterocephalus Palaemon (Pallas) [Online]
24 October 2016 © Peter Eeles Citation: Eeles, P. (2016). A Study of the Life Cycle of the Chequered Skipper Butterfly Carterocephalus palaemon (Pallas) [Online]. Available from http://www.dispar.org/reference.php?id=119 [Accessed October 24, 2016]. A Study of the Life Cycle of the Chequered Skipper Butterfly Carterocephalus palaemon (Pallas) Peter Eeles Abstract: Of all of the butterflies found in the British Isles, the complete life cycle of the Chequered Skipper is one of the most rarely observed, for several reasons. The first is that the distribution of the butterfly is restricted to north west Scotland where the level of recording is relatively low. The second is that, while many enthusiasts have made pilgrimages to see the adult butterfly, very few have put the same effort into locating the immature stages. Finally, the ecology of the butterfly requires the observer to put in a significant amount of time before they are rewarded with views of all stages. In this article, the author summarises his observations over a three year period, from 2014 to 2016. Introduction My first encounter with a Chequered Skipper (Carterocephalus palaemon) was on 2nd June 2006, when I visited Glasdrum National Nature Reserve (NNR), which is situated on the shores of Loch Creran in Argyllshire, Scotland. Despite the less-than-ideal weather, I managed to find two male butterflies roosting, closed-winged, among the raindrops. A return visit, two days later, proved to be much more fruitful and gave me my first opportunity to study the butterflies in more detail, in terms of both their appearance and behaviour.