Oviposition Selection in Montane Habitats, Biological Conservation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
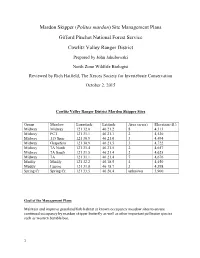
Mardon Skipper Site Management Plans
Mardon Skipper (Polites mardon) Site Management Plans Gifford Pinchot National Forest Service Cowlitz Valley Ranger District Prepared by John Jakubowski North Zone Wildlife Biologist Reviewed by Rich Hatfield, The Xerces Society for Invertebrate Conservation October 2, 2015 Cowlitz Valley Ranger District Mardon Skipper Sites Group Meadow Longitude Latitude Area (acres) Elevation (ft.) Midway Midway 121 32.0 46 21.2 8 4,313 Midway PCT 121 31.1 46 21.1 2 4,530 Midway 115 Spur 121 30.9 46 21.0 3 4,494 Midway Grapefern 121 30.9 46 21.5 3 4,722 Midway 7A North 121 31.4 46 21.5 2 4,657 Midway 7A South 121 31.5 46 21.4 2 4,625 Midway 7A 121 31.1 46 21.4 7 4,676 Muddy Muddy 121 32.2 46.18.5 4 4,450 Muddy Lupine 121 31.8 46 18.7 3 4,398 Spring Cr Spring Cr. 121 33.5 46 20.4 unknown 3,900 Goal of the Management Plans Maintain and improve grassland/forb habitat at known occupancy meadow sites to ensure continued occupancy by mardon skipper butterfly as well as other important pollinator species such as western bumble bee. 1 Introduction On the Gifford Pinchot National Forest (GPNF), mardon skippers were first detected on the Mt. Adams Ranger District (MTA) in 2000 and on Cowlitz Valley Ranger District (CVRD) in 2002. Mardon skippers are known to inhabit ten, upland dry grassy meadows on the CVRD. Portions of the meadows are mesic and are unsuitable mardon skipper habitat. -

Fasanbi SHOWCASE
Threatened Species Monitoring PROGRAMME Threatened Species in South Africa: A review of the South African National Biodiversity Institutes’ Threatened Species Programme: 2004–2009 Acronyms ADU – Animal Demography Unit ARC – Agricultural Research Council BASH – Big Atlassing Summer Holiday BIRP – Birds in Reserves Project BMP – Biodiversity Management Plan BMP-S – Biodiversity Management Plans for Species CFR – Cape Floristic Region CITES – Convention on International Trade in Endangered Species CoCT – City of Cape Town CREW – Custodians of Rare and Endangered Wildflowers CWAC – Co-ordinated Waterbird Counts DEA – Department of Environmental Affairs DeJaVU – December January Atlassing Vacation Unlimited EIA – Environmental Impact Assessment EMI – Environmental Management Inspector GBIF – Global Biodiversity Information Facility GIS – Geographic Information Systems IAIA – International Association for Impact Assessment IAIAsa – International Association for Impact Assessment South Africa IUCN – International Union for Conservation of Nature LAMP – Long Autumn Migration Project LepSoc – Lepidopterists’ Society of Africa MCM – Marine and Coastal Management MOA – memorandum of agreement MOU – memorandum of understanding NBI – National Botanical Institute NEMA – National Environmental Management Act NEMBA – National Environmental Management Biodiversity Act NGO – non-governmental organization NORAD – Norwegian Agency for Development Co–operation QDGS – quarter-degree grid square SABAP – Southern African Bird Atlas Project SABCA – Southern African -

Butterflies (Lepidoptera: Papilionoidea) in a Coastal Plain Area in the State of Paraná, Brazil
62 TROP. LEPID. RES., 26(2): 62-67, 2016 LEVISKI ET AL.: Butterflies in Paraná Butterflies (Lepidoptera: Papilionoidea) in a coastal plain area in the state of Paraná, Brazil Gabriela Lourenço Leviski¹*, Luziany Queiroz-Santos¹, Ricardo Russo Siewert¹, Lucy Mila Garcia Salik¹, Mirna Martins Casagrande¹ and Olaf Hermann Hendrik Mielke¹ ¹ Laboratório de Estudos de Lepidoptera Neotropical, Departamento de Zoologia, Universidade Federal do Paraná, Caixa Postal 19.020, 81.531-980, Curitiba, Paraná, Brazil Corresponding author: E-mail: [email protected]٭ Abstract: The coastal plain environments of southern Brazil are neglected and poorly represented in Conservation Units. In view of the importance of sampling these areas, the present study conducted the first butterfly inventory of a coastal area in the state of Paraná. Samples were taken in the Floresta Estadual do Palmito, from February 2014 through January 2015, using insect nets and traps for fruit-feeding butterfly species. A total of 200 species were recorded, in the families Hesperiidae (77), Nymphalidae (73), Riodinidae (20), Lycaenidae (19), Pieridae (7) and Papilionidae (4). Particularly notable records included the rare and vulnerable Pseudotinea hemis (Schaus, 1927), representing the lowest elevation record for this species, and Temenis huebneri korallion Fruhstorfer, 1912, a new record for Paraná. These results reinforce the need to direct sampling efforts to poorly inventoried areas, to increase knowledge of the distribution and occurrence patterns of butterflies in Brazil. Key words: Atlantic Forest, Biodiversity, conservation, inventory, species richness. INTRODUCTION the importance of inventories to knowledge of the fauna and its conservation, the present study inventoried the species of Faunal inventories are important for providing knowledge butterflies of the Floresta Estadual do Palmito. -

Implications for Conservation
Biodivers Conserv (2007) 16:4095–4107 DOI 10.1007/s10531-007-9209-z ORIGINAL PAPER Key traits in a threatened butterfly and its common sibling: implications for conservation Michael J. Samways Æ Shen-Shan Lu Received: 22 February 2007 / Accepted: 14 June 2007 / Published online: 20 July 2007 Ó Springer Science+Business Media B.V. 2007 Abstract We ask here which traits predispose one species to extreme rarity and possible extinction while a sympatric sibling is geographically widespread. With background knowledge on the level of habitat specialization of the two species, the population structure and movement of the localized and threatened Orachrysops ariadne were compared to those of the common and highly sympatric O. subravus, using mark-release-recapture. Of a total of 290 marked O. ariadne individuals 42.8% were recaptured, while of 631 O. subravus individuals 49.3% were recaptured. The Jolly-Seber model was used to estimate daily population numbers (Ni), survival rates (/i), recruitment rates (Bi), proportion of marked animals in the total population (µi), and the number of marked animals at risk (Mi). O. ariadne is a remarkably rare animal, averaging only 10 individuals haÀ1 within its small, remaining colonies. Average residence times of male adults were generally similar in both species, being just over 5 days. O. ariadne is a strong and rapid back and forth flier, covering mean recapture distances of 157 m, almost twice that of O. subravus, principally in search of scarce nectar sources. In short, the rarity of O. ariadne is not so much to do with behaviour, survivorship or longevity, but rather with limited availability of the spe- cialized habitat patches for both larvae and adults, and, in particular, the extreme scarcity of the host plant. -

Butterfly Conservation in Oak Savanna: Site Characterization, Nectar Resources, and the Effects of Management
BUTTERFLY CONSERVATION IN OAK SAVANNA: SITE CHARACTERIZATION, NECTAR RESOURCES, AND THE EFFECTS OF MANAGEMENT Lauren E. Yarrish A Thesis Submitted to the Graduate College of Bowling Green State University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE May 2011 Committee: Karen V. Root, Advisor Helen J. Michaels Jeffrey G. Miner ii ABSTRACT Karen V. Root, Advisor Oak savanna is a globally imperiled plant community characterized by scattered oak trees and an herbaceous ground layer. The Oak Openings Region in northwest Ohio was once dominated by oak savanna however, since European settlement the amount of oak savanna has been significantly reduced. Despite the large reduction in area, what remains continues to support high levels of biodiversity including several rare butterfly species such as the federally endangered Karner blue butterfly (Lycaeides melissa samuelis). To improve butterfly conservation efforts, this study sought to characterize and compare oak savanna sites, determine how butterflies utilize nectar resources, and assess the effects of land management practices in the Oak Openings Region. We conducted vegetation surveys at four oak savanna sites. Transects were established at each site and a quadrat frame was placed every 10 m along the transects. At each quadrat we recorded several factors known to be important to butterflies, such as flowering plant density, canopy cover, vegetation height etc. We also conducted opportunistic behavioral observations of butterflies, recording behavior at 10 minute intervals. Lastly, we obtained land management records from local agencies. Sites varied greatly with respect to the measured factors. Flowering plant densities were low compared to a previous study used to evaluate potential reintroduction sites for the Karner blue butterfly in northwest Ohio. -

Some Ecological Factors Influencing the Breeding Success of the Brenton Blue Butterfly, Orachrysops Niobe (Trimen) (Lepidoptera: Lycaenidae)
Edge.qxd 2005/12/09 10:02 Page 19 Some ecological factors influencing the breeding success of the Brenton Blue butterfly, Orachrysops niobe (Trimen) (Lepidoptera: Lycaenidae) D.A. EDGE Edge, D.A. 2002. Some ecological factors influencing the breeding success of the Bren- ton Blue butterfly, Orachrysops niobe (Trimen) (Lepidoptera: Lycaenidae). Koedoe 45(2): 19–34. Pretoria. ISSN 0075-6458. The Brenton Blue butterfly, Orachrysops niobe (Trimen, 1862) (Lepidoptera: Lycaenidae), is endemic to the southern Cape and is currently listed as Endangered. This study looks at some of the key ecological factors influencing the breeding success of the species—host plant abundance and condition, nectar sources, climate/ microclimate, and vegetation management techniques. The adult butterfly population was monitored over an entire breeding season; host plants were identified and individually monitored; and egg counts were done. This enabled the effects of a number of different manage- ment techniques to be evaluated (burning, cutting, physical removal of invasive ele- ments, and combinations thereof). A fivefold increase in the population of O. niobe was observed over the breeding season. This increase was positively correlated to a similar increase in host plant abundance in the areas where cutting and physical removal of invasive elements was practiced. Burning, by contrast, appeared to have a negative impact on host plant and butterfly abundance over the same period. Impacts of other fac- tors such as climate, nectar sources and the natural strength of the second brood are dis- cussed. A hypothesis, of megaherbivore activity as the principal historical disturbance mechanism promoting locally favourable conditions for O. niobe to establish and main- tain colonies, is proposed. -

Threatened Species PROGRAMME Threatened Species: a Guide to Red Lists and Their Use in Conservation LIST of ABBREVIATIONS
Threatened Species PROGRAMME Threatened Species: A guide to Red Lists and their use in conservation LIST OF ABBREVIATIONS AOO Area of Occupancy BMP Biodiversity Management Plan CBD Convention on Biological Diversity CITES Convention on International Trade in Endangered Species DAFF Department of Agriculture, Forestry and Fisheries EIA Environmental Impact Assessment EOO Extent of Occurrence IUCN International Union for Conservation of Nature NEMA National Environmental Management Act NEMBA National Environmental Management Biodiversity Act NGO Non-governmental Organization NSBA National Spatial Biodiversity Assessment PVA Population Viability Analysis SANBI South African National Biodiversity Institute SANSA South African National Survey of Arachnida SIBIS SANBI's Integrated Biodiversity Information System SRLI Sampled Red List Index SSC Species Survival Commission TSP Threatened Species Programme Threatened Species: A guide to Red Lists and their use in conservation OVERVIEW The International Union for Conservation of Nature (IUCN)’s Red List is a world standard for evaluating the conservation status of plant and animal species. The IUCN Red List, which determines the risks of extinction to species, plays an important role in guiding conservation activities of governments, NGOs and scientific institutions, and is recognized worldwide for its objective approach. In order to produce the IUCN Red List of Threatened Species™, the IUCN Species Programme, working together with the IUCN Species Survival Commission (SSC) and members of IUCN, draw on and mobilize a network of partner organizations and scientists worldwide. One such partner organization is the South African National Biodiversity Institute (SANBI), who, through the Threatened Species Programme (TSP), contributes information on the conservation status and biology of threatened species in southern Africa. -

Polites Mardon COMMON NA
U.S. FISH AND WILDLIFE SERVICE SPECIES ASSESSMENT AND LISTING PRIORITY ASSIGNMENT FORM SCIENTIFIC NAME: Polites mardon COMMON NAME: Mardon skipper LEAD REGION: Region 1 INFORMATION CURRENT AS OF: April 2007 STATUS/ACTION Species assessment ___ New candidate _X_ Continuing candidate ___ Non-petitioned _X_ Petitioned - Date petition received: 12/11/02 90-day positive - FR date: 12-month warranted but precluded - FR date: Did the petition request a reclassification of a listed species? FOR PETITIONED CANDIDATE SPECIES: a. Is listing warranted (if yes, see summary of threats below) YES b. To date, has publication of a proposal to list been precluded by other higher priority listing actions? YES c. If the answer to a. and b. is yes, provide an explanation of why the action is precluded. We find that the immediate issuance of a proposed rule and timely promulgation of a final rule for this species has been, for the preceding 12 months, and continues to be, precluded by higher priority listing actions (including candidate species with lower LPNs). During the past 12 months, almost our entire national listing budget has been consumed by work on various listing actions to comply with court orders and court- approved settlement agreements, meeting statutory deadlines for petition findings or listing determinations, emergency listing evaluations and determinations, and essential litigation-related, administrative, and program management tasks. We will continue to monitor the status of this species as new information becomes available. This review will determine if a change in status is warranted, including the need to make prompt use of emergency listing procedures. -

Management Plans for Mardon Skipper (Polites Mardon Ssp
Management Plans for mardon skipper (Polites mardon ssp. klamathensis) sites on Lily Glen and Howard Prairie Prepared by Rich Hatfield, Scott Hoffman Black, and Sarina Jepsen The Xerces Society for Invertebrate Conservation March 14, 2013 U.S.D.A. Forest Service Region 6 and U.S.D.I. Bureau of Land Management Interagency Special Status and Sensitive Species Program Table of Contents Section 1: Status and Threats ....................................................................................................... 3 Background ................................................................................................................................ 3 History and Taxonomy of Mardon Skipper in Southern Oregon ........................................... 3 Species Range, Distribution, Abundance, and Trends ............................................................ 4 Species Life History ................................................................................................................ 4 Recent Searches for Mardon Skipper in the Southern Oregon Cascades ............................... 6 Status of BLM sites in the southern Oregon Cascades ........................................................... 7 Howard Prairie and Lily Glen ................................................................................................. 8 Threats ....................................................................................................................................... 8 Small Populations .................................................................................................................. -

Community Planning and Economic Development Creating Solutions for Our Future Joshua Cummings, Director
COUNTY COMMISSIONERS John Hutchings District One Gary Edwards District Two Tye Menser District Three Community Planning and Economic Development Creating Solutions for Our Future Joshua Cummings, Director 2020 Thurston County Community Planning Field Screening Guidelines for Prairie Habitat Section 1 - 1.1 Purpose Under the development of the Habitat Conservation Plan (HCP), it is the long-term goal of Thurston County to conserve and restore large, intact areas of prairie habitat in addition to smaller tracts of land within 1/2 mi of larger prairies (Chapter 24.25.065 Thurston County Code (TCC)). While the screening process described in this protocol focuses on the detection of diagnostic prairie flora listed in the CAO, the overall intention for prairie conservation under the CAO and pending the HCP is to protect a much broader range of prairie butterflies, birds and mammals, and habitat. South Puget Sound Prairie ecosystems support a wide range of rare flora and fauna, some of which are listed under federal or state protection, including butterflies which are considered Species of Conservation Concern (SCC) or Greatest Conservation Need (SGCN). Particular attention is given to the protection of federally listed and imperiled butterfly species in Thurston County, such as the Taylor’s checkerspot (TCB, Euphydryas editha taylori), Puget blue (Icaria icarioides blackmorei), hoary elfin (Callophrys polios), Oregon branded skipper (Hesperia Colorado oregonia), Mardon skipper (Polites mardon), and valley silverspot (Speyeria zerene) butterflies, and the plant species known to serve as host and nectar plants for these butterflies. Other federally listed and candidate prairie species include the streaked horned lark (Eromophila alpestris strigata), Mazama pocket gopher (Thomomys mazama), and the Oregon vesper sparrow (Pooecetes gramineus). -

Species Fact Sheet Mardon Skipper Polites Mardon Current and Historical Status
Species Fact Sheet Mardon Skipper Polites mardon Photo Credit: Holmes, Conboy NWR STATUS: CANDIDATE Mardon skipper potentially occurs in these Washington counties: Skamania, Yakima, Klickitat, Pierce, and Thurston (Map may reflect historical as well as recent sightings) The Mardon skipper butterfly, Polites mardon, was designated a federal candidate species in 1999. Current and Historical Status The historical status of the Mardon skipper is not precisely known. Abundance estimates and distribution for this species prior to 1980 are nonexistent. This species most likely declined with the decline of early seral grassland habitat. Post 1980, mardon skippers were historically collected from four widely separated locations: the south Puget Sound region of Washington, the southern Washington Cascades, the Siskiyou Mountains of southern Oregon, and coastal northern California. Prior to 1980, very little information was known about Mardon skippers. It was thought to be endemic to Washington State only. Interest in the species has led to additional surveys and improved information on the species distribution. By 2000, this species was known to occur at more than 130 sites located in south Puget Sound and the Mt. Adams area in Washington; the Siskiyou Mountains in southern Oregon; and Del Norte County, California. All populations are relatively small with few populations supporting more than 50 individuals. In Washington, this species has been observed and is regularly surveyed on south Puget Sound prairies and on grassland openings in a forest matrix in the southern Cascades of Washington. As recently as 2009, only 2 populations were detected on south Puget Sound prairies, one at Scatter Creek Wildlife Area in Thurston County and one on the Artillery Impact Area at Fort Lewis, Pierce County. -

Management Plans for All Southern Oregon Cascade Mardon Skipper
Management Plans for all Southern Oregon Cascade Mardon Skipper (Polites mardon) sites on the Bureau of Land Management’s Hunter Creek Area of Critical Environmental Concern (ACEC) Prepared by Rich Hatfield, Scott Hoffman Black, and Sarina Jepsen, The Xerces Society for Invertebrate Conservation March 14, 2013 U.S.D.A. Forest Service Region 6 and U.S.D.I. Bureau of Land Management Interagency Special Status and Sensitive Species Program 1 Table of Contents Section 1: Status and Threats ....................................................................................................... 3 Background ................................................................................................................................ 3 History and Taxonomy of Mardon skipper in Southern Oregon ....................................... 3 Species Life History ............................................................................................................... 4 Recent Searches for Mardon Skipper. .................................................................................. 6 Status on the Hunter Creek ACEC ........................................................................................ 7 Threats ....................................................................................................................................... 8 Small Populations .................................................................................................................. 8 Climate Change .....................................................................................................................